
| Tracks 1-19 |
| Track 1 |
Clinical decision-making in the use of adjuvant therapy for patients with Stage II CRC |
| Track 2 |
Counseling patients about the risks and benefits of adjuvant therapy |
| Track 3 |
Use of adjuvant capecitabine
monotherapy for patients with
lower-risk disease |
| Track 4 |
Monitoring patients for oxaliplatin- associated neuropathy |
| Track 5 |
Dosing capecitabine monotherapy
in the adjuvant setting |
| Track 6 |
Patient follow-up after adjuvant
therapy |
| Track 7 |
Management of potentially curable
mCRC |
| Track 8 |
Treatment algorithm for resectable
versus unresectable mCRC |
| Track 9 |
Utilization of response to preoperative
therapy as an in vivo test of
chemosensitivity |
| Track 10 |
Therapeutic approach to
unresectable mCRC and an
asymptomatic, synchronous
primary tumor |
|
| Track 11 |
Avoidance of surgical intervention
for patients with intact,
asymptomatic primary tumors |
| Track 12 |
Selection of appropriate patients
for first-line therapy with a
fluoropyrimidine bevacizumab
treatment platform |
| Track 13 |
Addition of bevacizumab to
FOLFOX versus FOLFIRI for the
treatment of mCRC |
| Track 14 |
Use of treatment holidays in the
management of mCRC |
| Track 15 |
Dosing of bevacizumab |
| Track 16 |
Continuation of bevacizumab after
disease progression: The iBET
trial |
| Track 17 |
Treatment after progression on
first-line FOLFOX/bevacizumab |
| Track 18 |
Reintroduction of oxaliplatin after
discontinuation due to drug-related
toxicity |
| Track 19 |
Clinical trial endpoints in the
evaluation of biologic and targeted
agents |
|
|
Select Excerpts from the Interview
Tracks 1, 3
 DR LOVE:
DR LOVE: What do you see as some of the key current issues in the use of
adjuvant therapy for patients with colorectal cancer?
 DR HURWITZ: The biggest questions regarding chemotherapy in the
adjuvant setting have to do with stage, namely lymph node-positive versus node-negative disease, and to some degree, the existence of any additional
risk factors (eg, an inadequate number of lymph nodes). The debate surrounds
whether patients with lymph node-negative disease or those with Dukes B2 — or Stage II — cancer benefit from therapy. I usually address that issue by
having a long discussion with the patient. I explain that the relative benefit
for these patients is probably similar, but because the absolute risk is less, the
absolute benefit is also smaller, but it still may exist.
DR HURWITZ: The biggest questions regarding chemotherapy in the
adjuvant setting have to do with stage, namely lymph node-positive versus node-negative disease, and to some degree, the existence of any additional
risk factors (eg, an inadequate number of lymph nodes). The debate surrounds
whether patients with lymph node-negative disease or those with Dukes B2 — or Stage II — cancer benefit from therapy. I usually address that issue by
having a long discussion with the patient. I explain that the relative benefit
for these patients is probably similar, but because the absolute risk is less, the
absolute benefit is also smaller, but it still may exist.
 DR LOVE: How do you approach the treatment of patients with lower-risk
disease, and where does capecitabine fit in?
DR LOVE: How do you approach the treatment of patients with lower-risk
disease, and where does capecitabine fit in?
 DR HURWITZ: Settings arise in which patients have preexisting neuropathy or
have careers or lifestyles for which neuropathy would be a major quality-of-life
adjustment. I’ll let them know that they have two options: FOLFOX, which
probably has a slightly superior outcome, or capecitabine as monotherapy — or
if for some reason that would not be available, 5-FU is probably better than
nothing from the point of view of disease control and survival.
DR HURWITZ: Settings arise in which patients have preexisting neuropathy or
have careers or lifestyles for which neuropathy would be a major quality-of-life
adjustment. I’ll let them know that they have two options: FOLFOX, which
probably has a slightly superior outcome, or capecitabine as monotherapy — or
if for some reason that would not be available, 5-FU is probably better than
nothing from the point of view of disease control and survival.
 DR LOVE: You mentioned the option of capecitabine monotherapy. How do
you dose in that setting?
DR LOVE: You mentioned the option of capecitabine monotherapy. How do
you dose in that setting?
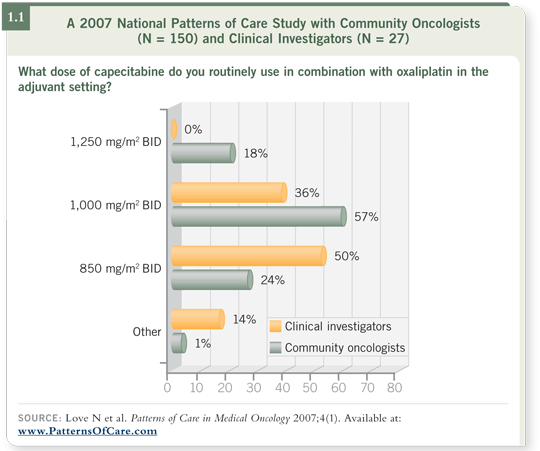
 DR HURWITZ: For monotherapy, most patients in the US will begin with
1,000 mg/m2 twice daily for two out of three weeks (1.1). The dose will be
increased if they don’t have problems in the first cycle. For patients with an excellent performance status, it is reasonable to start at the full 1,250 mg/m2 twice daily.
DR HURWITZ: For monotherapy, most patients in the US will begin with
1,000 mg/m2 twice daily for two out of three weeks (1.1). The dose will be
increased if they don’t have problems in the first cycle. For patients with an excellent performance status, it is reasonable to start at the full 1,250 mg/m2 twice daily.
Tracks 12-13
 DR LOVE:
DR LOVE: How do you go about choosing first-line systemic therapy for
metastatic disease?
 DR HURWITZ: The main issue in the metastatic setting is whether the patient
is a candidate for systemic therapy. The second issue is the presence of any
major contraindications to the backbones of therapy, which would be either a
fluoropyrimidine or bevacizumab. A quick family history will tell you if any
family member has had problems receiving chemotherapy. If a family member
has a dihydropyrimidine dehydrogenase (DPD) deficiency and could not
metabolize pyrimidines, usually everybody in the family has been alerted.
DR HURWITZ: The main issue in the metastatic setting is whether the patient
is a candidate for systemic therapy. The second issue is the presence of any
major contraindications to the backbones of therapy, which would be either a
fluoropyrimidine or bevacizumab. A quick family history will tell you if any
family member has had problems receiving chemotherapy. If a family member
has a dihydropyrimidine dehydrogenase (DPD) deficiency and could not
metabolize pyrimidines, usually everybody in the family has been alerted.
Another issue would be bevacizumab-specific contraindications. Recent (six
months to one year) arteriovascular complications — myocardial infarction,
stroke or active disease — are fairly significant contraindications to bevacizumab.
 DR LOVE: Is the use of bevacizumab as beneficial with an oxaliplatin-containing
regimen as it is with an irinotecan-based regimen?
DR LOVE: Is the use of bevacizumab as beneficial with an oxaliplatin-containing
regimen as it is with an irinotecan-based regimen?
 DR HURWITZ: I don’t believe it’s an issue of oxaliplatin versus irinotecan. In
the second-line FOLFOX study from Dr Giantonio (ECOG-E3200; [Giantonio
2007]) — FOLFOX/bevacizumab versus FOLFOX alone versus bevacizumab
monotherapy — the benefits in terms of response rates and survival
with the addition of bevacizumab to second-line FOLFOX were significant.
The improvements using bevacizumab were as large as those in any other study,
and the second-line setting probably includes a harder-to-treat population.
DR HURWITZ: I don’t believe it’s an issue of oxaliplatin versus irinotecan. In
the second-line FOLFOX study from Dr Giantonio (ECOG-E3200; [Giantonio
2007]) — FOLFOX/bevacizumab versus FOLFOX alone versus bevacizumab
monotherapy — the benefits in terms of response rates and survival
with the addition of bevacizumab to second-line FOLFOX were significant.
The improvements using bevacizumab were as large as those in any other study,
and the second-line setting probably includes a harder-to-treat population.
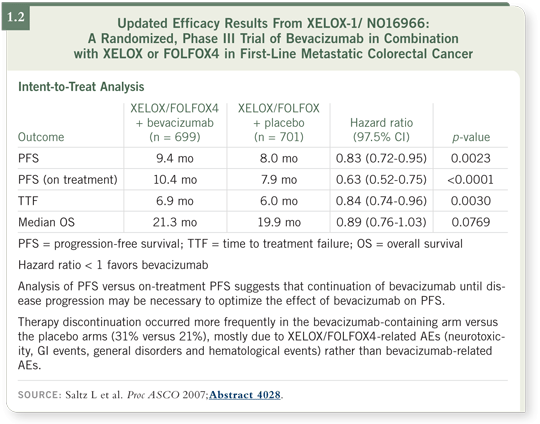
A comparison of other data to those of the first-line NO16966 study of
bevacizumab with oxaliplatin (Saltz 2007) is confounded by several clinical
study variables. The FOLFOX/CAPOX/bevacizumab data (1.2) do not appear
as positive as those using the bolus IFL regimen. However, bolus IFL is not the
best platform to use in the first-line treatment of colorectal cancer.
Track 14
 DR LOVE:
DR LOVE: How do you maximize treatment benefit from oxaliplatin in
metastatic disease?
 DR HURWITZ: One approach is to be preemptive through the use of a calendar
schedule. This is the OPTIMOX (Tournigand 2006) approach, by which
stopping and starting treatment are based as much on the calendar as they are
on the patient’s symptoms or disease control.
DR HURWITZ: One approach is to be preemptive through the use of a calendar
schedule. This is the OPTIMOX (Tournigand 2006) approach, by which
stopping and starting treatment are based as much on the calendar as they are
on the patient’s symptoms or disease control.
I find that adjustment based on the patient’s symptoms — as long as the
threshold of symptoms is lowered — ends up being a nearly identical approach.
I have a bias to try to adjust based on how the patient is faring rather than the calendar, but in practice, the two approaches are most likely similar.
 DR LOVE: When you stop oxaliplatin, do you continue the 5-FU or
capecitabine and the bevacizumab?
DR LOVE: When you stop oxaliplatin, do you continue the 5-FU or
capecitabine and the bevacizumab?
 DR HURWITZ: Yes, currently my algorithm is to reduce or stop only the
problem agent and to continue the portions of therapy that seem to help, as
long as they’re well tolerated.
DR HURWITZ: Yes, currently my algorithm is to reduce or stop only the
problem agent and to continue the portions of therapy that seem to help, as
long as they’re well tolerated.
For patients who need a break for personal reasons, or for asthenia, I believe
stopping even the fluoropyrimidine and bevacizumab for a period of several
weeks to two months is a reasonable approach, as long as the disease burden and
patient symptoms allow for the holiday.
Track 16
 DR LOVE:
DR LOVE: What are your thoughts regarding whether or not to continue
bevacizumab for patients with disease progression?
 DR HURWITZ: If the disease clearly progresses on therapy, that therapy, whatever
it is, should be stopped and patients should receive whatever other options seem
reasonable. The difficulty — particularly if progression is slow — is knowing
whether the slow progression is attributable to bevacizumab or to indolent
disease. That’s why we have to use our best judgment until we see better data.
DR HURWITZ: If the disease clearly progresses on therapy, that therapy, whatever
it is, should be stopped and patients should receive whatever other options seem
reasonable. The difficulty — particularly if progression is slow — is knowing
whether the slow progression is attributable to bevacizumab or to indolent
disease. That’s why we have to use our best judgment until we see better data.
The cooperative groups are running a study known as iBET (1.3). This
will address the question of whether patients fare better with bevacizumab
continued into that “second-line” setting and whether they respond better to
the 5-mg versus the 10-mg dose.

Select Publications

