| Tracks 1-12 |
| Track 1 |
Strategies to ameliorate oxaliplatin-associated neurotoxicity |
| Track 2 |
Combined Oxaliplatin Neurotoxicity
Prevention Trial (CONcePT)
to optimize first-line FOLFOX with
bevacizumab |
| Track 3 |
Preliminary analysis of CONcePT: Decreased response to FOLFOX/ bevacizumab in patients receiving calcium/magnesium |
| Track 4 |
Implications of the CONcePT results |
| Track 5 |
Updated efficacy and survival results with adjuvant FOLFOX in the MOSAIC trial |
| Track 6 |
Lymph node sampling in
colorectal cancer (CRC) |
| Track 7 |
Development of molecular tests to aid in treatment decision-making for CRC |
|
| Track 8 |
BRiTE: Exposure to bevacizumab beyond first progression and overall survival among patients with metastatic CRC |
| Track 9 |
Intergroup trial SWOG-S0600 (iBET): Irinotecan-based chemotherapy and cetuximab with or without bevacizumab after disease progression on bevacizumab-containing first-line therapy |
| Track 10 |
Treatment with bevacizumab beyond disease progression |
| Track 11 |
Implications of the negative
PACCE trial results with combined
bevacizumab and panitumumab |
| Track 12 |
Potential explanation for the PACCE trial results |
|
|
Select Excerpts from the Interview
Tracks 2-4
 DR LOVE:
DR LOVE: Can you review the recent findings from the CONcePT trial?
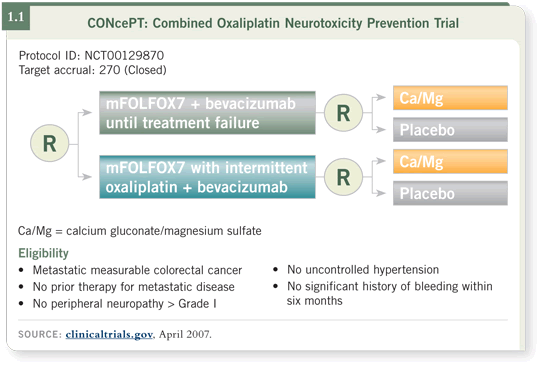
 DR GROTHEY: The CONcePT trial was designed to optimize the use of
FOLFOX with bevacizumab as first-line therapy for patients with advanced
colorectal cancer. CONcePT stands for “Combined Oxaliplatin Neurotoxicity
Prevention Trial.”
DR GROTHEY: The CONcePT trial was designed to optimize the use of
FOLFOX with bevacizumab as first-line therapy for patients with advanced
colorectal cancer. CONcePT stands for “Combined Oxaliplatin Neurotoxicity
Prevention Trial.”
By “Combined,” we mean to approach this toxicity issue from two different
angles: First, we use calcium/magnesium in a placebo-controlled comparison,
and second, we use the stop-and-go approach for oxaliplatin in analogy to
the OPTIMOX trials in France (Tournigand 2006; Maindrault-Goebel 2007;
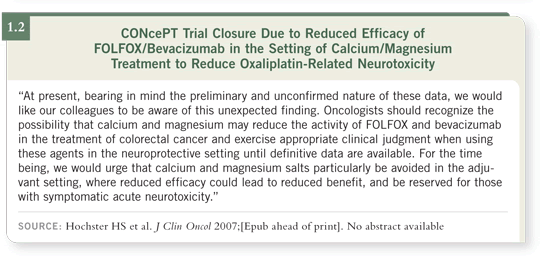
[1.1]). The Data Monitoring Committee halted the CONcePT trial in May 2007. We were notified on June 15, 2007 that the trial had to be permanently
closed (1.2). All the data are based on a preliminary interim analysis, so there
are a number of caveats to keep in mind.
The fact that the trial was permanently closed by the Data Monitoring
Committee shows that we are not justified to continue the trial, so we need to
step back and see what’s happening.
In the preliminary analysis, the use of calcium/magnesium was apparently
associated with a significantly decreased response rate compared to placebo.
Patients who received calcium/magnesium as potentially neuroprotective
therapy had a significantly lower response rate than patients who were on the
placebo arm (1.2). This is something we cannot ignore.
Tracks 8-10
 DR LOVE:
DR LOVE: Can you review the findings from the BRiTE Tumor Registry
(Grothey 2007)?
 DR GROTHEY: The BRiTE Registry was founded in February 2004 when
bevacizumab was approved as a component of treatment for first-line
metastatic colorectal cancer. Later, the approval was extended to the second
line, but when bevacizumab became approved, we had limited clinical experience
with the drug. We had data from one pivotal trial (Hurwitz 2004).
Certain toxicities like GI perforations were reported, and later, atherothrombotic
events were recognized, though only in a small number of patients.
DR GROTHEY: The BRiTE Registry was founded in February 2004 when
bevacizumab was approved as a component of treatment for first-line
metastatic colorectal cancer. Later, the approval was extended to the second
line, but when bevacizumab became approved, we had limited clinical experience
with the drug. We had data from one pivotal trial (Hurwitz 2004).
Certain toxicities like GI perforations were reported, and later, atherothrombotic
events were recognized, though only in a small number of patients.
The idea of the BRiTE registry was to obtain information on a larger number
of patients — eventually it was 1,953 patients — enrolled in a “real-life” setting.
Oncologists who use bevacizumab in combination with whatever chemotherapy
regimen they deem appropriate document the clinical course of their patients
over a long period of time. We are developing a nice database on these patients.
When patients experienced their first progression on therapy, some physicians
continued bevacizumab and some did not continue bevacizumab in combination
with a different treatment regimen. Outcomes, progressive disease and
overall survival data were documented in the BRiTE registry. Of the 1,953
patients, approximately 1,450 experienced progressive disease, and some groups
did not receive any further therapy because of poor performance status. Some
patients continued therapy without bevacizumab, and some patients continued
bevacizumab in combination with other chemotherapies.
This is a nonrandomized setting, but we tried to compare therapies within
the BRiTE registry — the outcomes for patients who continued bevacizumab
and those who did not. The effects were quite profound because patients who
continued bevacizumab had a remarkably longer overall survival than the
patients who did not receive bevacizumab (1.3).
This may be related to the fact that physicians decided to continue bevacizumab
only for patients who had a better performance status or who had experienced a better response with prior therapy. Having said that, we tried
to account for all of these factors in a multivariate analysis by considering age,
performance status, the number of metastatic sites, some laboratory analysis,
duration of first-line therapy, et cetera. Still, the continuation of bevacizumab
beyond progression turned out to be a significant factor in this analysis.
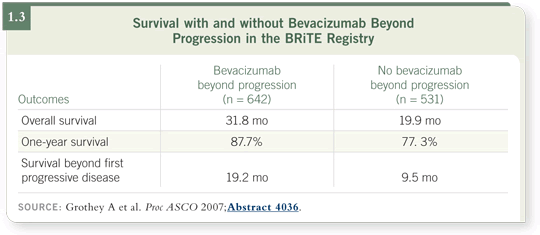
If there appears to be a profound effect, such as a difference in overall survival
of 31 months versus 19 months using bevacizumab beyond progression
(Grothey 2007), we need to validate this in a prospective clinical trial.
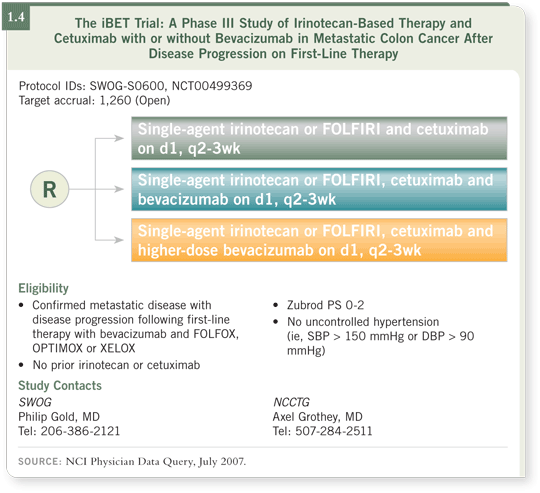
The so-called iBET trial (SWOG/NCCTG/NCIC iBET S0600), which
started on June 15th of this year, is an Intergroup trial randomly assigning
patients who already received a bevacizumab-containing first-line therapy
with oxaliplatin — either FOLFOX or XELOX — to second-line treatment
with FOLFIRI or irinotecan in combination with cetuximab followed by
bevacizumab at either 5 mg/kg or 10 mg/kg every two weeks (1.4).
So patients on two of the three arms will receive bevacizumab beyond
progression. I believe this is one of the most important trials we’re running in
colorectal cancer because a considerable number of our physicians use bevacizumab
beyond progression — 40 to 50 percent are using it as we speak.
 DR LOVE: How did the results from the BRiTE registry affect your own
clinical decision-making?
DR LOVE: How did the results from the BRiTE registry affect your own
clinical decision-making?
 DR GROTHEY: In my clinical practice, I’ve used both approaches. I’ve treated
patients with bevacizumab beyond progression, and I’ve stopped bevacizumab
upon progression. Outside of a clinical trial, I individualize therapy based on
the effect of therapy on patients in the first-line setting.
DR GROTHEY: In my clinical practice, I’ve used both approaches. I’ve treated
patients with bevacizumab beyond progression, and I’ve stopped bevacizumab
upon progression. Outside of a clinical trial, I individualize therapy based on
the effect of therapy on patients in the first-line setting.
For instance, if a patient receives FOLFOX/bevacizumab as front-line therapy
— which many of our patients receive right now in clinical practice — I
routinely stop oxaliplatin after about four months and try to maintain the
response with 5-FU/bevacizumab. For some patients it works well, and we
see the tumor stabilize for more than a year or a year and a half — as long as
we’ve had experience with bevacizumab. Then you see this slow creeping up
of metastases, though it’s never a rapid progression that would suggest it is a
completely useless therapy.
 DR LOVE: What fraction of BRiTE patients had prior responses?
DR LOVE: What fraction of BRiTE patients had prior responses?
 DR GROTHEY: No imbalance was indicated among patients who had a clinical
complete response on therapy in the first-line setting. Prior response was one
of the factors we tested in the multivariate analysis, and this did not influence
whether or not physicians continued bevacizumab.
DR GROTHEY: No imbalance was indicated among patients who had a clinical
complete response on therapy in the first-line setting. Prior response was one
of the factors we tested in the multivariate analysis, and this did not influence
whether or not physicians continued bevacizumab.
Select publications

