
| Tracks 1-15 |
| Track 1 |
Phase II study of neoadjuvant chemoradiation therapy with bevacizumab for rectal cancer |
| Track 2 |
Outcome of local excision after preoperative chemoradiation therapy among selected patients with T3 rectal cancer |
| Track 3 |
Potential mechanisms of action of anti-VEGF therapy |
| Track 4 |
Potential effects of bevacizumab on tumor cell signaling |
| Track 5 |
Pragmatic physician and patient considerations in the use of capecitabine versus infusional 5-FU |
| Track 6 |
X-ACT trial: Efficacy of adjuvant capecitabine versus bolus 5-FU/leucovorin |
| Track 7 |
Tolerability of capecitabine-based chemoradiation therapy |
| Track 8 |
Capecitabine and the timing of radiation therapy during preoperative chemoradiation for rectal cancer |
|
| Track 9 |
Incorporation of oxaliplatin
into chemoradiation therapy
for rectal cancer |
| Track 10 |
Toxicity with the addition of oxaliplatin to chemoradiation therapy |
| Track 11 |
Neoadjuvant study of chemoradiation therapy with capecitabine, erlotinib and bevacizumab for rectal cancer |
| Track 12 |
Minimization of radiation therapy for patients with rectal cancer |
| Track 13 |
Prognostic factors impacting treatment decision-making in rectal cancer |
| Track 14 |
Clinical management of T1-2
rectal tumors |
| Track 15 |
Biopsy of anal cancer following
chemoradiation therapy |
|
|
Select Excerpts from the Interview
Track 5
 DR LOVE:
DR LOVE: What led to the decision to use capecitabine, as opposed to
continuous infusion 5-FU, in your rectal cancer study of neoadjuvant
radiation therapy and bevacizumab?
 DR CRANE: It was primarily the tolerability and ease of delivery of
capecitabine. The optimal way to administer either infusional 5-FU or
capecitabine with radiation therapy is to make dosage adjustments for Grade II
toxicities, such as diarrhea or nausea. With infusional 5-FU, we would interrupt the therapy and allow the patient a couple of days for it to resolve. Once
it resolved, we would start back with a 25 percent dose reduction.
DR CRANE: It was primarily the tolerability and ease of delivery of
capecitabine. The optimal way to administer either infusional 5-FU or
capecitabine with radiation therapy is to make dosage adjustments for Grade II
toxicities, such as diarrhea or nausea. With infusional 5-FU, we would interrupt the therapy and allow the patient a couple of days for it to resolve. Once
it resolved, we would start back with a 25 percent dose reduction.
In addition, the patients had to have a line put in, and many patients didn’t
like that.
The best way to minimize toxicity is to modulate the dose based on how
the patient tolerates the therapy. Capecitabine revolutionized the number of
personnel we needed to accomplish that goal.
Tracks 6-7
 DR LOVE:
DR LOVE: Can you discuss the NSABP-R-04 rectal cancer study, which
is evaluating neoadjuvant radiation therapy with capecitabine versus 5-FU,
both regimens with or without oxaliplatin?
 DR CRANE: When using any radiosensitizer in rectal cancer, efficacy is the
most important consideration. How do we prove the efficacy of a radiosensitizer?
Basically, we’re using neoadjuvant chemotherapy and radiation therapy
together, which have independent toxicities. We want to use systemic doses
of chemotherapy, if we can do so safely, with radiation therapy. From that
perspective, the X-ACT study adjuvant data (Twelves 2005) provide enough
information to administer capecitabine with radiation therapy.
DR CRANE: When using any radiosensitizer in rectal cancer, efficacy is the
most important consideration. How do we prove the efficacy of a radiosensitizer?
Basically, we’re using neoadjuvant chemotherapy and radiation therapy
together, which have independent toxicities. We want to use systemic doses
of chemotherapy, if we can do so safely, with radiation therapy. From that
perspective, the X-ACT study adjuvant data (Twelves 2005) provide enough
information to administer capecitabine with radiation therapy.
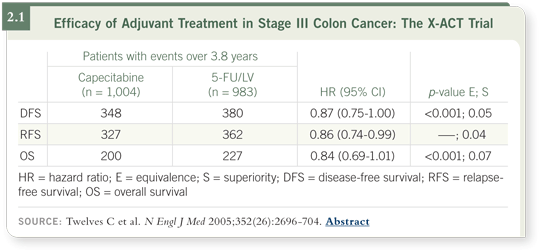
X-ACT was a randomized trial comparing 5-FU/leucovorin to capecitabine,
primarily in European patients with node-positive colon cancer. Patients
receiving capecitabine showed a relapse-free survival benefit, a strong trend
toward a disease-free survival benefit and no difference in overall survival
(Twelves 2005; [2.1]).
The trial was powered for noninferiority, but it showed strong trends toward
superiority for capecitabine, with less toxicity except for hand-foot syndrome.
That was a dose-limiting toxicity, but as in all the other trials, patients
receiving capecitabine experienced less hematologic and gastrointestinal
toxicity (Twelves 2005).
I believe the potential exists to reduce the toxicity because if capecitabine is
optimally managed when Grade II toxicity occurs — meaning that it’s interrupted
and then adjusted by a 25 percent dose reduction — you can avoid
Grade III toxicity. In fact, we have less than a five percent incidence of Grade
III toxicities.
We did a matched-pair analysis, published last year, of infusional 5-FU versus
capecitabine in combination with radiation therapy. We had approximately
90 patients in each group, and they were matched for T and N stage. The
patients receiving capecitabine had less low-grade mucositis and diarrhea with
a slightly numerically improved pathologic CR rate (Das 2006).
So the use of capecitabine is something we’ve adopted as a general platform
from which to incorporate novel targeted agents with chemoradiation therapy
regimens in upper and lower gastrointestinal cancer — liver, hepatobiliary, pancreatic and anal tumors. We are now also running a CAPOX trial for anal
cancer.
Track 9
 DR LOVE:
DR LOVE: Another strategy incorporated into NSABP-R-04 is the use of
oxaliplatin as part of a neoadjuvant regimen. What do we know about that?
 DR CRANE: Oxaliplatin has been proven in the adjuvant setting, which makes
it interesting to use with radiation therapy. That’s why it’s in NSABP-R-04.
Its value may be that it’s a radiosensitizer or that it’s being used earlier in the
treatment. It’s a worthwhile drug to study in the neoadjuvant setting, and
NSABP-R-04 was improved when they added oxaliplatin as the second part of
a two-by-two randomization.
DR CRANE: Oxaliplatin has been proven in the adjuvant setting, which makes
it interesting to use with radiation therapy. That’s why it’s in NSABP-R-04.
Its value may be that it’s a radiosensitizer or that it’s being used earlier in the
treatment. It’s a worthwhile drug to study in the neoadjuvant setting, and
NSABP-R-04 was improved when they added oxaliplatin as the second part of
a two-by-two randomization.
I believe it’s acceptable to use oxaliplatin-based chemoradiation therapy off
protocol if it’s done selectively, carefully and with close monitoring because the
rates of Grade III gastrointestinal toxicity, mainly diarrhea, will be higher.
Select publications

