
| Tracks 1-10 |
| Track 1 |
Development of the MOSAIC trial of adjuvant FOLFOX in Stage II and IIl colon cancer |
| Track 2 |
Updated six-year efficacy results, including survival, of the MOSAIC trial |
| Track 3 |
Benefit of the addition of adjuvant oxaliplatin for patients with Stage II disease |
| Track 4 |
Clinical management of
oxaliplatin-associated neuropathy |
| Track 5 |
Comparison of MOSAIC and
NSABP-C-07 outcomes |
| Track 6 |
AVANT: Adjuvant FOLFOX versus
FOLFOX or XELOX with bevacizumab
in colon cancer |
|
| Track 7 |
Considerations in the design
and execution of new adjuvant
clinical trials in colon cancer |
| Track 8 |
OPTIMOX trials: Evaluation of
“treatment holidays” in
metastatic disease |
| Track 9 |
BRiTE study: Overall survival
with the use of bevacizumab
beyond disease progression in
metastatic CRC |
| Track 10 |
OPTIMOX-3/DREAM (Double
inhibition, Reintroduction,
Erlotinib, Avastin, Metastatic
CRC) study |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: Can you review the updated data you presented from the
MOSAIC trial at the 2007 ASCO meeting?
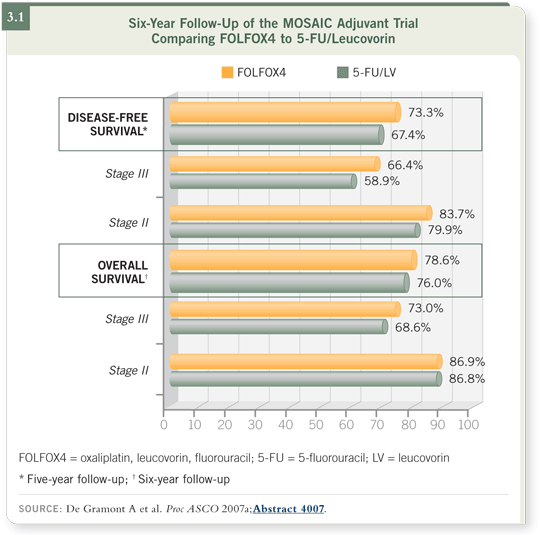
 DR DE GRAMONT: Fortunately, the disease-free survival advantage that was
observed for FOLFOX4 at three years’ follow-up was confirmed at five years.
For the first time, I presented the overall survival data with a minimum of six
years’ follow-up in all patients, and the benefit was observed only in patients
with Stage III colorectal cancer (de Gramont 2007a; [3.1]).
DR DE GRAMONT: Fortunately, the disease-free survival advantage that was
observed for FOLFOX4 at three years’ follow-up was confirmed at five years.
For the first time, I presented the overall survival data with a minimum of six
years’ follow-up in all patients, and the benefit was observed only in patients
with Stage III colorectal cancer (de Gramont 2007a; [3.1]).
Among patients with Stage II disease, no benefit in survival is evident. The
population was a mix of patients with low-risk and high-risk disease. We
could define an advantage in the high-risk population in terms of disease-free
survival, but even in this population we could not observe a survival advantage.
However, the numbers were small and these were exploratory analyses.
It’s good news that patients with Stage II colorectal cancer — high-risk and
low-risk disease — in the MOSAIC trial have a six-year probability of survival
of 87 percent.
 DR LOVE: What was the relapse rate in patients with Stage II disease?
DR LOVE: What was the relapse rate in patients with Stage II disease?
 DR DE GRAMONT: The overall relapse rate was nearly 10 percent, and a 3.8
percent difference in disease-free survival between the two treatment arms
existed.
DR DE GRAMONT: The overall relapse rate was nearly 10 percent, and a 3.8
percent difference in disease-free survival between the two treatment arms
existed.
Track 8
 DR LOVE:
DR LOVE: Can you discuss how you currently approach the patient with
metastatic disease in a nonprotocol setting?
 DR DE GRAMONT: Outside of a protocol, my management strategy will be
similar to OPTIMOX1. I start with the modified FOLFOX7 regimen, which
is an optimized regimen that has a much lower toxicity than FOLFOX4. I administer bevacizumab to patients who meet the same inclusionary criteria
we used in the clinical trials.
DR DE GRAMONT: Outside of a protocol, my management strategy will be
similar to OPTIMOX1. I start with the modified FOLFOX7 regimen, which
is an optimized regimen that has a much lower toxicity than FOLFOX4. I administer bevacizumab to patients who meet the same inclusionary criteria
we used in the clinical trials.
Bevacizumab is the drug that has most increased the progression-free survival
in these patients. We learned from the NO16966 study that it’s important not to
stop bevacizumab (Cassidy 2007; Saltz 2007). When you decide to start treatment
with bevacizumab, patients know they will continue on bevacizumab.
The results presented by Axel Grothey demonstrated that survival is much
better in the patients who continue on bevacizumab after progression (Grothey
2007).
 DR LOVE: For a patient whom you have started on FOLFOX7 and bevacizumab,
do you plan ahead that you will stop the oxaliplatin at a certain point?
DR LOVE: For a patient whom you have started on FOLFOX7 and bevacizumab,
do you plan ahead that you will stop the oxaliplatin at a certain point?
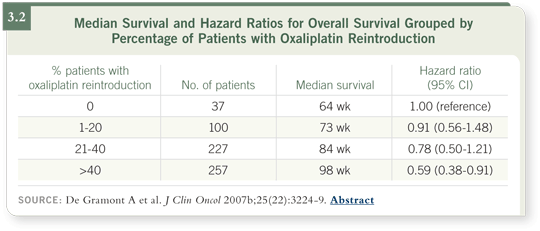
 DR DE GRAMONT: Yes, oxaliplatin is stopped at six cycles. I’m sure that if
I stop at six cycles, the patient will have no neuropathy. When the patient
progresses, I can reintroduce oxaliplatin, and in the OPTIMOX1 study the
median survival was 21 months. In our centers in which more than 40 percent
of the patients had oxaliplatin reintroduction, the median survival was more
than two years (3.2).
DR DE GRAMONT: Yes, oxaliplatin is stopped at six cycles. I’m sure that if
I stop at six cycles, the patient will have no neuropathy. When the patient
progresses, I can reintroduce oxaliplatin, and in the OPTIMOX1 study the
median survival was 21 months. In our centers in which more than 40 percent
of the patients had oxaliplatin reintroduction, the median survival was more
than two years (3.2).
In the OPTIMOX2 trial, the median survival was 26 months in the arm
without continuation of bevacizumab (Maindrault-Goebel 2007). So the stop-and-go strategy for oxaliplatin is my strategy for all patients with advanced
disease. If we add bevacizumab, it will be administered with FOLFOX,
between the administrations of FOLFOX and continued with the same
administration of FOLFOX.
Track 9
 DR LOVE:
DR LOVE: Do you ever continue bevacizumab at disease progression,
particularly for a patient who has had a good response to treatment with
FOLFOX?
 DR DE GRAMONT: Data presented from the BRiTE trial are provocative
(Grothey 2007; [1.3]), and I will fully support the iBET trial, which plans to evaluate whether we should continue bevacizumab. On one arm, patients will
receive second-line therapy, which will include irinotecan/cetuximab. On the
other arm, patients will receive irinotecan/cetuximab combined with bevacizumab.
DR DE GRAMONT: Data presented from the BRiTE trial are provocative
(Grothey 2007; [1.3]), and I will fully support the iBET trial, which plans to evaluate whether we should continue bevacizumab. On one arm, patients will
receive second-line therapy, which will include irinotecan/cetuximab. On the
other arm, patients will receive irinotecan/cetuximab combined with bevacizumab.
The data from the BRiTE registry are impressive. We have 30-month survival
— an impressive survival from progression to death in this group of patients. I
cannot imagine a bias that could explain this huge difference between patients
who did not receive bevacizumab at progression and the others. I believe that
when oncologists see these data, they will want to continue bevacizumab.
Select publications

