
| Tracks 1-8 |
| Track 1 |
Bevacizumab as a potential radiation sensitizer in rectal cancer |
| Track 2 |
Clinical trial of bevacizumab alone and concurrent with chemoradiation therapy in rectal cancer |
| Track 3 |
Direct evidence of antivascular effects of bevacizumab in rectal cancer |
| Track 4 |
Clinical response to neoadjuvant
chemoradiation with bevacizumab
in rectal cancer |
|
| Track 5 |
Capecitabine versus infusional 5-FU as neoadjuvant therapy in rectal cancer |
| Track 6 |
Pathologic complete response with neoadjuvant combined chemoradiation and targeted therapies |
| Track 7 |
Addition of oxaliplatin to neoadjuvant chemoradiation therapy for rectal cancer |
| Track 8 |
American College of Surgeons trial of local excision in patients with T2 rectal cancer |
|
|
Select Excerpts from the Interview
Track 1
 DR LOVE:
DR LOVE: Would you discuss the background of your trial that evaluated
bevacizumab as part of neoadjuvant treatment of rectal cancer?
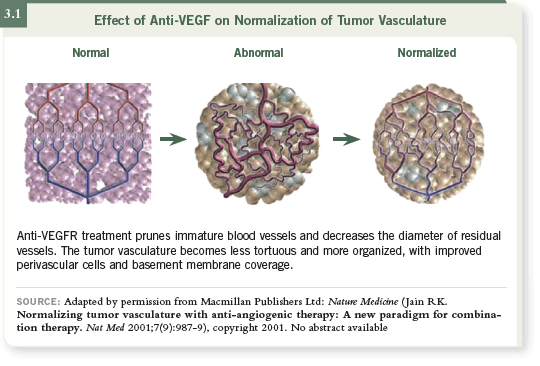
 DR WILLETT: Dr Rakesh Jain had been interested in a hypothesis called
“normalization” (Jain 2001; [3.1]), which he examined in preclinical models.
DR WILLETT: Dr Rakesh Jain had been interested in a hypothesis called
“normalization” (Jain 2001; [3.1]), which he examined in preclinical models.
The hypothesis is that the tumor vasculature is highly inefficient and is associated
with high levels of interstitial pressure and hypoxia. So if you administer
anti-angiogenic agents — specifically agents targeting VEGF — these agents
may work not only through direct blood vessel killing but also by improving
the efficiency of the remaining tumor vasculature.
 DR LOVE: What was known about bevacizumab and radiation sensitization?
DR LOVE: What was known about bevacizumab and radiation sensitization?
 DR WILLETT: The preclinical work (Lee 2000; Yuan 1996) in a variety of
mouse models demonstrated that if bevacizumab was administered with radiation
therapy, the amount of radiation needed to control the tumors was less
than with radiation therapy alone.
DR WILLETT: The preclinical work (Lee 2000; Yuan 1996) in a variety of
mouse models demonstrated that if bevacizumab was administered with radiation
therapy, the amount of radiation needed to control the tumors was less
than with radiation therapy alone.
Track 2
 DR LOVE:
DR LOVE: How did you choose the study design for your trial of bevacizumab
and chemoradiation therapy for patients with rectal cancer?
 DR WILLETT: We wanted to observe the effect of bevacizumab as a single
agent on rectal cancer before introducing radiation therapy and 5-FU.
DR WILLETT: We wanted to observe the effect of bevacizumab as a single
agent on rectal cancer before introducing radiation therapy and 5-FU.
According to the trial design (Willett 2004), patients received a single infusion
of bevacizumab prior to the introduction of 5-FU and radiation therapy
with concurrent bevacizumab. We were keenly interested in what would be
happening at a relatively short period after the first bevacizumab infusion.
At day 12, typically, after the first bevacizumab infusion, evaluations (flexible
sigmoidoscopies, biopsies, interstitial fluid pressure, functional imaging,
serum/blood assays) were repeated in terms of the correlative studies.
So it provided an opportunity to observe a human malignancy in vivo after
bevacizumab treatment, and it allowed an opportunity to see the resulting
types of effects.
 DR LOVE: How long did patients receive the chemoradiation therapy and
bevacizumab?
DR LOVE: How long did patients receive the chemoradiation therapy and
bevacizumab?
 DR WILLETT: The protocol design was as follows: An infusion of bevacizumab
was administered on day one, and two weeks later a second infusion of bevacizumab
was followed by the introduction of pelvic irradiation and continuous
infusion 5-FU.
DR WILLETT: The protocol design was as follows: An infusion of bevacizumab
was administered on day one, and two weeks later a second infusion of bevacizumab
was followed by the introduction of pelvic irradiation and continuous
infusion 5-FU.
We administered a standard course of radiation therapy — 50.4 Gray over 5.5
weeks. A seven-day continuous infusion of 5-FU at 225 mg/m2 was administered
throughout the course of radiation therapy.
Bevacizumab was administered every other week for a total of four infusions
of bevacizumab with 50 Gray of radiation and 5-FU during the course of
external-beam radiation therapy. Surgery was performed seven to nine weeks
after completion of the bevacizumab to allow for clearance of the drug,
considering the half-life of bevacizumab.
Track 3
 DR LOVE:
DR LOVE: Can you review the findings of your study?
 DR WILLETT: The initial Phase I portion (Willett 2004) of the trial included
six patients who received the first dose of bevacizumab in the trial, which was
5 mg/kg. At day 12, after the first infusion of bevacizumab, our first patient
underwent a flexible sigmoidoscopy and appeared to show a response with the
monotherapy alone.
DR WILLETT: The initial Phase I portion (Willett 2004) of the trial included
six patients who received the first dose of bevacizumab in the trial, which was
5 mg/kg. At day 12, after the first infusion of bevacizumab, our first patient
underwent a flexible sigmoidoscopy and appeared to show a response with the
monotherapy alone.
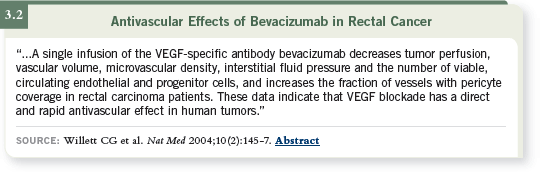
We did not run into any dose-limiting toxicity. However, when we began
to put the data together for the correlative studies, we noted some interesting
findings (3.2).
One such finding was that the interstitial fluid pressure in these patients had
dropped from baseline to day 12, a finding that perfectly matched the results
that Dr Jain had observed in the xenograft models.
 DR LOVE: You mentioned what happened to the first patient after two weeks
— what about the other five patients?
DR LOVE: You mentioned what happened to the first patient after two weeks
— what about the other five patients?
 DR WILLETT: Essentially, disease remained in the other five patients. Tumors
seemed, in terms of response, about the same in size — no big changes. It is
interesting that some of the tumors became perhaps a little more pale on gross
visualization. We did see a drop in the tumor blood flow with perfusion CT
scans.
DR WILLETT: Essentially, disease remained in the other five patients. Tumors
seemed, in terms of response, about the same in size — no big changes. It is
interesting that some of the tumors became perhaps a little more pale on gross
visualization. We did see a drop in the tumor blood flow with perfusion CT
scans.
According to the 18-fluorodeoxyglucose PET scans, essentially no difference
had appeared in standardized uptake values between pretreatment and day
12. We also saw a drop in microvessel density between baseline and day 12,
consistent with preclinical work.
You might ask whether a drop in tumor blood flow goes against the normalization
hypothesis. Probably not — remember, the perfusion CT is a relatively gross
measure. Even with a drop in blood flow, the level of tumor metabolic activity
remained the same, which, in fact, suggested some element of normalization.
Track 4
 DR LOVE:
DR LOVE: What were the clinical responses in the initial six patients at
surgery?
 DR WILLETT: In five of the six patients, we saw a flat ulcer in the surgical
specimen with no exophytic or macroscopic disease. One patient had gross
disease remaining. When these specimens were sectioned and examined histologically,
we typically observed a nest of cells admixed into a deep fibrous
tissue.
DR WILLETT: In five of the six patients, we saw a flat ulcer in the surgical
specimen with no exophytic or macroscopic disease. One patient had gross
disease remaining. When these specimens were sectioned and examined histologically,
we typically observed a nest of cells admixed into a deep fibrous
tissue.
 DR LOVE: In those five patients, if you had to make a guess, what fraction of
the tumor do you think was destroyed?
DR LOVE: In those five patients, if you had to make a guess, what fraction of
the tumor do you think was destroyed?
 DR WILLETT: That is a hard question. We used various grading scales to try to
correlate the amount of residual disease with what one would have expected
pretreatment. The clinical responses were excellent, with an ulcer remaining,
and microscopic disease remained.
DR WILLETT: That is a hard question. We used various grading scales to try to
correlate the amount of residual disease with what one would have expected
pretreatment. The clinical responses were excellent, with an ulcer remaining,
and microscopic disease remained.
The next cohort of patients received bevacizumab at a higher dose level of 10
mg/kg. Five patients were assigned to that dose level. Two of the five patients
who received the higher dose level showed complete pathological responses
— that is, absolutely no malignant cells were left in the surgical specimens.
The other three patients also showed good responses, but again, microscopic
disease remained. Note that these 11 patients were assessed by one pathologist,
who “bread-loafed” the specimens individually, so the stringency of the
pathological examination of these specimens was probably as tight as could be.
Track 5
 DR LOVE:
DR LOVE: What are your thoughts about the controversy regarding
capecitabine versus continuous infusion 5-FU as neoadjuvant therapy for
rectal cancer?
 DR WILLETT: The need to address the question in a Phase III trial as neoadjuvant
treatment for rectal carcinoma is clear. Many clinicians have adopted
capecitabine as an alternative to infusional 5-FU regimens, and not only for
rectal carcinoma.
DR WILLETT: The need to address the question in a Phase III trial as neoadjuvant
treatment for rectal carcinoma is clear. Many clinicians have adopted
capecitabine as an alternative to infusional 5-FU regimens, and not only for
rectal carcinoma.
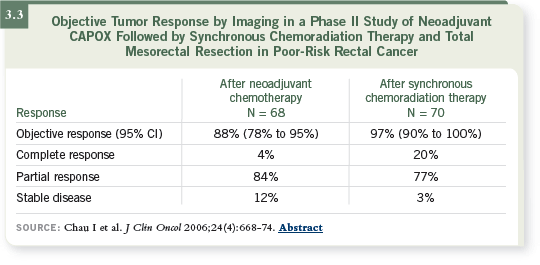
The data from Phase I and II studies (Chau 2006; Glynne-Jones 2005) of
capecitabine and radiation therapy (3.3) suggest that it is as beneficial as 5-FU
infusions, with a slightly different toxicity profile. We have used it, but we
also discuss the option carefully with the patient.
Select Publications

