
| Tracks 1-13 |
| Track 1 |
Recent developments in the treatment of metastatic colorectal cancer |
| Track 2 |
Emerging clinical data with panitumumab |
| Track 3 |
Recent clinical trials of first-line therapy in metastatic colorectal cancer |
| Track 4 |
XELOX-1/NO16966: CAPOX or FOLFOX4 with or without bevacizumab as first-line therapy |
| Track 5 |
Investigations of chemotherapy holidays in the treatment of metastatic disease |
| Track 6 |
Doublet antibodies in the treatment of metastatic colorectal cancer |
| Track 7 |
Side effects and tolerability of the anti-EGFR antibody panitumumab |
|
| Track 8 |
Current role of panitumumab in the treatment of metastatic colorectal cancer |
| Track 9 |
Predictors of response to anti-EGFR antibodies |
| Track 10 |
Clinical trial strategies incorporating biologic agents in the adjuvant setting |
| Track 11 |
Use of chemotherapy with biologic agents to render hepatic metastases resectable |
| Track 12 |
Safety of bevacizumab and its role in the adjuvant setting |
| Track 13 |
Identification of targets for biologic agents and the individualization of therapy |
|
|
Select Excerpts from the Interview
Track 4
 DR LOVE:
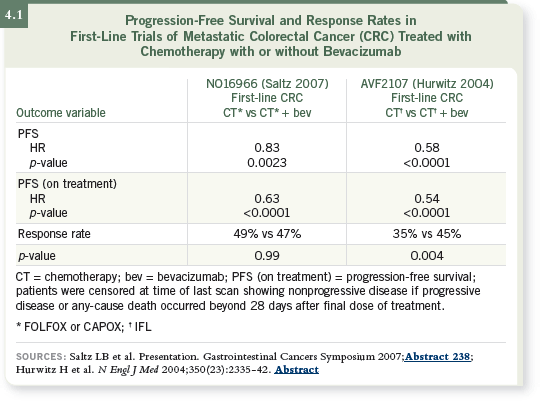
DR LOVE: Can you discuss the results of “Trial 66,” recently reported by
Cassidy and Saltz (Cassidy 2007; Saltz 2007; [4.1])?
 DR HECHT: Initially, that study compared CAPOX and FOLFOX, then
bevacizumab was included and it became a two-by-two design. The investigators
did reach their intended endpoint, demonstrating that CAPOX was not
inferior to FOLFOX. In fact, the two arms were very similar.
DR HECHT: Initially, that study compared CAPOX and FOLFOX, then
bevacizumab was included and it became a two-by-two design. The investigators
did reach their intended endpoint, demonstrating that CAPOX was not
inferior to FOLFOX. In fact, the two arms were very similar.
In evaluating progression-free survival, they examined fluoropyrimidine/oxaliplatin-containing chemotherapy with or without bevacizumab, and
a statistically significant improvement was observed, but it was not of the expected magnitude. In fact, in an unplanned subgroup analysis, no PFS
improvement was seen with FOLFOX, but it was evident when the CAPOX
and FOLFOX arms were evaluated together. The hazard ratio was approximately
0.83. A fairly small improvement in median survival was seen.
So one of the questions that has arisen is, why are these results different than
when FOLFOX with bevacizumab was evaluated in the second-line setting?
Did the Europeans treat their patients differently? I’m anxious to see what
additional data will be presented.
 DR LOVE: In the IFL/bevacizumab study, when patients experienced toxicity
from the chemotherapy, the chemotherapy was stopped and bevacizumab was
continued until progression (Hurwitz 2004; [4.1]). In the 66 study, however,
chemotherapy and bevacizumab were discontinued when patients developed
toxicity, so patients ended up receiving less bevacizumab.
DR LOVE: In the IFL/bevacizumab study, when patients experienced toxicity
from the chemotherapy, the chemotherapy was stopped and bevacizumab was
continued until progression (Hurwitz 2004; [4.1]). In the 66 study, however,
chemotherapy and bevacizumab were discontinued when patients developed
toxicity, so patients ended up receiving less bevacizumab.
 DR HECHT: That has been one of the interpretations of the data. The problem
is, the data are the data. The question is, why are the data the data?
DR HECHT: That has been one of the interpretations of the data. The problem
is, the data are the data. The question is, why are the data the data?
Track 10
 DR LOVE:
DR LOVE: Where are we right now with regard to colorectal cancer trials
in the adjuvant setting evaluating bevacizumab?
 DR HECHT: Two trials are under way. One is the NSABP trial evaluating
bevacizumab with a fluoropyrimidine and oxaliplatin for six months followed
by six additional months of bevacizumab.
DR HECHT: Two trials are under way. One is the NSABP trial evaluating
bevacizumab with a fluoropyrimidine and oxaliplatin for six months followed
by six additional months of bevacizumab.
The other is the worldwide, three-arm AVANT trial (4.2), which compares
FOLFOX as the standard, FOLFOX with bevacizumab or CAPOX with
bevacizumab. That trial was closed for a few months because of a question
regarding increased toxicity in one of the arms. However, it was felt that it
was not sufficient to change the trial. Additional monitoring was incorporated,
and it will be years before we receive adjuvant data. Other targeted therapies
are being evaluated, such as antibodies, and I believe they will form the next
wave of adjuvant trials.

Select Publications

