
| Tracks 1-12 |
| Track 1 |
Incorporating drug “holidays” in the paradigm of treating metastatic colorectal cancer |
| Track 2 |
Case discussion: A patient with a good response to FOLFOX/ bevacizumab with multiple treatment holidays |
| Track 3 |
Chemotherapy before surgery in potentially curative settings |
| Track 4 |
Current research and treatment
issues in rectal cancer |
| Track 5 |
Clinical approach to adjuvant therapy for patients with rectal cancer |
| Track 6 |
Clinical trials incorporating EGFR inhibitors as first-line therapy |
|
| Track 7 |
Continuation of bevacizumab after disease progression |
| Track 8 |
Use of panitumumab for patients with advanced colorectal cancer |
| Track 9 |
Major ongoing adjuvant clinical
trials in colon cancer |
| Track 10 |
Patient acceptance of rash as a side effect of adjuvant therapy |
| Track 11 |
EVEREST: Cetuximab dose escalation study for patients with metastatic colorectal cancer and no or slight skin reactions to cetuximab standard dose treatment |
| Track 12 |
Evolving data on capecitabine with oxaliplatin in the adjuvant and metastatic settings |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: What are your thoughts about using treatment holidays in
metastatic disease?
 DR VENOOK: I’ve always been a proponent of drug holidays without any basis
for it — I just rely on common sense and try to do the best thing possible for
my patients.
DR VENOOK: I’ve always been a proponent of drug holidays without any basis
for it — I just rely on common sense and try to do the best thing possible for
my patients.
One of my patients is approximately four years into his metastatic disease. He
initially responded to front-line FOLFOX/bevacizumab therapy and then
developed neuropathy. We gave him six months off the treatment. During
that time, his disease progressed a bit and the neuropathy was persistent. We
switched to FOLFIRI and bevacizumab at the time of progression.
 DR LOVE: So you stopped everything — is that what you normally do?
DR LOVE: So you stopped everything — is that what you normally do?
 DR VENOOK: This was some years ago, and we stopped everything for that
patient. Now my instinct is to continue the components of therapy without
the oxaliplatin.
DR VENOOK: This was some years ago, and we stopped everything for that
patient. Now my instinct is to continue the components of therapy without
the oxaliplatin.
This patient was switched to FOLFIRI/bevacizumab and had another
response, which then peaked. His neuropathy resolved, and his disease
progressed.
We went back to FOLFOX and bevacizumab, and he responded again. He’s
four years out now on cetuximab and responding.
Track 6
 DR LOVE:
DR LOVE: What are some of the current clinical trials that you anticipate
will have the greatest impact on clinical practice over the next few years?
 DR VENOOK: A study that has not yet opened but will be important to clinical
practice patterns is the iBET trial (SWOG/NCCTG/NCIC iBET S0600).
It will evaluate whether to continue bevacizumab for patients experiencing
progression on first-line chemotherapy and bevacizumab.
DR VENOOK: A study that has not yet opened but will be important to clinical
practice patterns is the iBET trial (SWOG/NCCTG/NCIC iBET S0600).
It will evaluate whether to continue bevacizumab for patients experiencing
progression on first-line chemotherapy and bevacizumab.
Patients experiencing progression on FOLFOX/bevacizumab will be
randomly assigned to either irinotecan/cetuximab or irinotecan/cetuximab
and bevacizumab.
This trial will approach the biggest question I’m asked regularly: “Do you
continue bevacizumab or don’t you continue bevacizumab at the time of
progression?”
 DR LOVE: How do you approach that sort of situation outside of a protocol?
DR LOVE: How do you approach that sort of situation outside of a protocol?
 DR VENOOK: Generally, our instinct is to not continue bevacizumab, although
I’ll admit we often reintroduce it later on.
DR VENOOK: Generally, our instinct is to not continue bevacizumab, although
I’ll admit we often reintroduce it later on.
For a 30-year-old whose risk of stroke or myocardial infarction I believe to be
sufficiently low, I might keep it going. For a 70-year-old with whom I suspect
I’ve already tempted fate, I might stop. I don’t have a one-size-fits-all answer.
 DR LOVE: Do you factor in how the patient’s tumor is responding to the
therapy?
DR LOVE: Do you factor in how the patient’s tumor is responding to the
therapy?
 DR VENOOK: Yes, although it can cut either way. For a patient who shows a
dramatic response but whose treatment is only palliative, you could argue to
stop the bevacizumab because you’ve already obtained considerable value out
of it.
DR VENOOK: Yes, although it can cut either way. For a patient who shows a
dramatic response but whose treatment is only palliative, you could argue to
stop the bevacizumab because you’ve already obtained considerable value out
of it.
The other way to look at it is, don’t stop the bevacizumab because it may be
contributing to the response. So it’s a gray area. There’s very little black and
white about the decision.
Track 9
 DR LOVE:
DR LOVE: What adjuvant studies are you currently enrolling patients on?
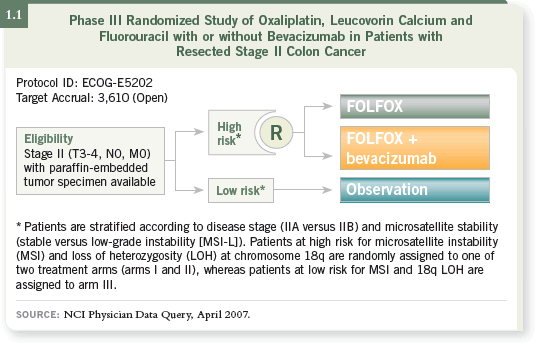
 DR VENOOK: We enroll patients on ECOG-E5202, which I believe is an
incredibly important study. Patients with Stage II disease are risk stratified
based on the molecular features of their cancer (1.1).
DR VENOOK: We enroll patients on ECOG-E5202, which I believe is an
incredibly important study. Patients with Stage II disease are risk stratified
based on the molecular features of their cancer (1.1).
Patients at low risk, who are expected to comprise about 60 percent of the
patients, are observed. Patients at high risk — deletion on 18q, microsatellite
stability — receive FOLFOX or FOLFOX/bevacizumab.
I believe that’s such an absolutely important study to distinguish who
doesn’t need chemotherapy and to see whether the data hold up. It would be
wonderful to save so many patients from exposure to chemotherapy.
We’re also participating in the Intergroup study (CALGB-80203), evaluating
FOLFOX or FOLFIRI with or without cetuximab (Venook 2006).
Track 12
 DR LOVE:
DR LOVE: Would you discuss the evolving data on the use of capecitabine
in combination with oxaliplatin in the adjuvant and metastatic settings?
 DR VENOOK: In the adjuvant setting, we’re not there yet. We do have data
“in the hopper” — the AVANT trial. In the next year we hope to have
information that might tell us it’s safe, and at least equivalent, to substitute
capecitabine for 5-FU.
DR VENOOK: In the adjuvant setting, we’re not there yet. We do have data
“in the hopper” — the AVANT trial. In the next year we hope to have
information that might tell us it’s safe, and at least equivalent, to substitute
capecitabine for 5-FU.
Dr Cassidy conducted a study in the metastatic setting (NO16966) that is
complex (Cassidy 2006). It was initially a CAPOX versus FOLFOX regimen.
Then it was amended to be CAPOX with bevacizumab or placebo versus
FOLFOX with bevacizumab or placebo.
The overall take-home message is that CAPOX does not appear to be inferior
to FOLFOX in the advanced disease setting. I believe we can take that to the
bank, except this was almost entirely a European trial.
The dose of capecitabine in this trial, 1 g/m2 BID with oxaliplatin, is a larger
dose than we use in the United States. A lot of data suggest, for not entirely
certain reasons, that you can’t dose patients in the United States at the same
dose of capecitabine that you can use in Europe.
So with the caveat that you have to ensure that the dosing is okay, I believe
CAPOX is not inferior to FOLFOX.
With the addition of bevacizumab, overall, patients did better. Progression-free
survival was improved. However, it is curious that the incremental benefit
of bevacizumab wasn’t nearly as large as it has been with treatments in other
colon cancer studies.
 DR LOVE: What about the use of capecitabine alone in the adjuvant setting?
DR LOVE: What about the use of capecitabine alone in the adjuvant setting?
 DR VENOOK: We use it occasionally. For a patient who isn’t a candidate for
FOLFOX — very elderly with comorbidities, neuropathy and the sort of red
flags seen with oxaliplatin — we’ll use capecitabine alone.
DR VENOOK: We use it occasionally. For a patient who isn’t a candidate for
FOLFOX — very elderly with comorbidities, neuropathy and the sort of red
flags seen with oxaliplatin — we’ll use capecitabine alone.
Select Publications

