
| Tracks 1-16 |
| Track 1 |
Introduction |
| Track 2 |
XELOX-1/NO16966: CAPOX or FOLFOX4 with or without bevacizumab as first-line therapy |
| Track 3 |
Continuation of therapy until progression on a first-line trial of IFL with or without bevacizumab |
| Track 4 |
Impact of the addition of bevacizumab to chemotherapy on response rate |
| Track 5 |
Clinical implications of XELOX-1/ NO16966 and the use of CAPOX |
| Track 6 |
Evaluating alternative schedules of capecitabine |
| Track 7 |
Clinical implications of XELOX-1/ NO16966 and the use of bevacizumab in combination with first-line chemotherapy |
| Track 8 |
Use of chemotherapy holidays |
| Track 9 |
Safety of a 10-minute infusion of bevacizumab |
|
| Track 10 |
Phase I study of oral agent S-1 in combination with oxaliplatin and bevacizumab in patients with advanced solid tumors |
| Track 11 |
Evaluation of routine anti-histamine premedication after the first two doses of cetuximab |
| Track 12 |
Geographic variation in cetuximab-associated infusion reactions |
| Track 13 |
Pharmacokinetics of cetuximab administered every two weeks |
| Track 14 |
Studies evaluating chemotherapy/ bevacizumab with or without tyrosine kinase EGFR inhibitors |
| Track 15 |
Predictors of response to tyrosine kinase EGFR inhibitors |
| Track 16 |
Impact of physical activity on colon cancer recurrence and survival |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: Can you talk about the background of the XELOX-1/NO16966 study that you presented at the ASCO GI Cancers Symposium?
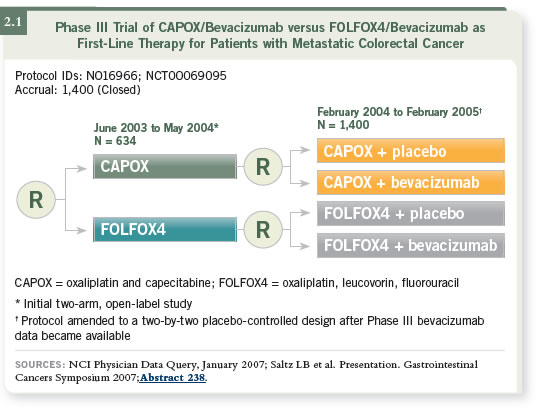
 DR SALTZ: XELOX-1/NO16966 was a study that Jim Cassidy and I led
together (Saltz 2007; Cassidy 2007). It started out as a straightforward comparison
of CAPOX and FOLFOX in the first-line metastatic setting and was designed to be a noninferiority study. Shortly after we began accrual, new data
emerged indicating that bevacizumab added a clinically meaningful benefit to
IFL chemotherapy (Hurwitz 2004).
DR SALTZ: XELOX-1/NO16966 was a study that Jim Cassidy and I led
together (Saltz 2007; Cassidy 2007). It started out as a straightforward comparison
of CAPOX and FOLFOX in the first-line metastatic setting and was designed to be a noninferiority study. Shortly after we began accrual, new data
emerged indicating that bevacizumab added a clinically meaningful benefit to
IFL chemotherapy (Hurwitz 2004).
Those findings caused us to rethink our study and repackage it as a two-by-two
randomization, in which patients were randomly assigned to CAPOX or
FOLFOX first, then to that regimen combined with either placebo or bevacizumab
(2.1).
The study had two primary endpoints, with progression-free survival being
the target in both cases. One endpoint was noninferiority of CAPOX
versus FOLFOX, and Jim Cassidy presented those data at the 2007 ASCO
GI Symposium (Cassidy 2007). At that meeting, I discussed the superiority
question of adding bevacizumab to front-line oxaliplatin-based chemotherapy
(Saltz 2007). The end of the story is that it was a positive study.
The primary endpoint of progression-free survival was improved with the
addition of bevacizumab to front-line oxaliplatin-based therapy. The p-value
was 0.0023, and the hazard ratio was 0.83. The incremental improvement was
1.4 months. This confirms the original study of IFL with or without bevacizumab
that Dr Hurwitz published (Hurwitz 2004; [2.2]).
However, the study was not as positive as we had hoped it would be in two
respects. First, we would have liked to see more improvement from the
addition of bevacizumab than we did. Second, we would have liked to see
patients in all the arms do better than they did.
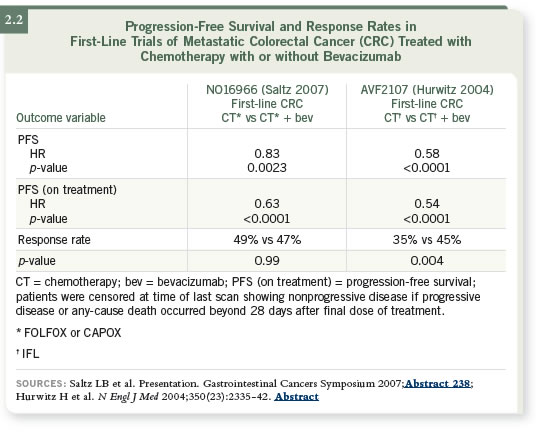
We had a sense that if we took FOLFOX, which was a superior regimen to
IFL, and added bevacizumab, we would start to see a median target progression-
free survival of around one year, and we did not see that in the study.
The median progression-free survival was 9.4 months. So bevacizumab helps,
it is appropriate to add to front-line therapy and it improves progression-free
survival in that population, but we wanted to understand why the differences
were more modest than we’d hoped.
This difference is, in all likelihood, accountable by early discontinuation of
chemotherapy before progression in patients in our study. When we consider
what happened on this trial, more than half of the patients discontinued
for reasons other than progression or death, and our best hypothesis is that
much of this discontinuation was due to what are likely to be oxaliplatin-based
known toxicities — neurotoxicity, primarily — and although the study
permitted investigators to discontinue oxaliplatin and continue the fluoropy-rimidines
and the bevacizumab, most of the investigators didn’t do that.
What we see in the study is that when oxaliplatin stopped, everything stopped
— and everything stopped several months before progression or death.
 DR LOVE: What about in the Hurwitz trial?
DR LOVE: What about in the Hurwitz trial?
 DR SALTZ: In the Hurwitz study, when people stopped, it was almost always
for either progression or death. I believe that what this indicates is that the
oxaliplatin-based regimens are a bit more subtle and require careful understanding to be used in their optimal sense. In order to obtain the most benefit
from the addition of bevacizumab, we would hypothesize that it is important
to continue both the bevacizumab and whatever active chemotherapy drugs
are tolerable until progression.
DR SALTZ: In the Hurwitz study, when people stopped, it was almost always
for either progression or death. I believe that what this indicates is that the
oxaliplatin-based regimens are a bit more subtle and require careful understanding to be used in their optimal sense. In order to obtain the most benefit
from the addition of bevacizumab, we would hypothesize that it is important
to continue both the bevacizumab and whatever active chemotherapy drugs
are tolerable until progression.
We see a big difference in terms of how many patients received treatment up
until progression on this trial versus the Hurwitz study, and we hypothesize
that it may largely account for the more modest benefit seen in progression-free
survival.
 DR LOVE: It makes sense when you think about it — if you continue the
fluoropyrimidine and bevacizumab, you would delay progression compared to
stopping everything. What fraction of patients were entered from the United
States in your trial versus in Hurwitz’s?
DR LOVE: It makes sense when you think about it — if you continue the
fluoropyrimidine and bevacizumab, you would delay progression compared to
stopping everything. What fraction of patients were entered from the United
States in your trial versus in Hurwitz’s?
 DR SALTZ: The Hurwitz study was predominantly a United States study. Our
study accrued 90 percent outside the US.
DR SALTZ: The Hurwitz study was predominantly a United States study. Our
study accrued 90 percent outside the US.
Track 5
 DR LOVE:
DR LOVE: What are the clinical implications of the study?
 DR SALTZ: First, the study satisfies me regarding the noninferiority of
CAPOX versus FOLFOX. That does not mean everybody should run out and
use CAPOX — it means it is an option that can be considered and that may
be appropriate for some patients.
DR SALTZ: First, the study satisfies me regarding the noninferiority of
CAPOX versus FOLFOX. That does not mean everybody should run out and
use CAPOX — it means it is an option that can be considered and that may
be appropriate for some patients.
I believe capecitabine is a good alternative for motivated patients who can be
counted on to take their medications, to be aware of toxicity, to hold their
medication, to contact their treating physician if toxicity develops, to get the
numbers right, to not miss a dose, to not double up on doses and to adhere to
the regimen of 14 days on, seven days off.
I still tend to favor either FOLFOX or FOLFIRI, which I use to equal
degrees, as my front-line cytotoxic regimen. However, previously, I would tell
people that I haven’t seen data to tell me that CAPOX is an acceptable alternative,
but now I have.
Track 7
 DR LOVE:
DR LOVE: What are the clinical implications of the bevacizumab data
from XELOX-1/NO16966?
 DR SALTZ: The study confirms prior studies that showed bevacizumab
increases progression-free survival (Giantonio 2005; Hurwitz 2004). I believe
it justifies my continued feeling that bevacizumab is an appropriate component
of first-line chemotherapy except for patients that have a significant contraindication,
such as a history of significant arterial thrombotic events, serious
wound-healing issues and so on.
DR SALTZ: The study confirms prior studies that showed bevacizumab
increases progression-free survival (Giantonio 2005; Hurwitz 2004). I believe
it justifies my continued feeling that bevacizumab is an appropriate component
of first-line chemotherapy except for patients that have a significant contraindication,
such as a history of significant arterial thrombotic events, serious
wound-healing issues and so on.
As with other trials, it indicates nothing about whether bevacizumab should be
continued in multiple lines of therapy. The revised package insert for bevacizumab
says it is approved for first- or second-line therapy — it does not say
first and second-line therapy — so it is my practice to use bevacizumab in one
line of therapy. I use it in first-line therapy unless there is a contraindication.
If that contraindication is resolved so that bevacizumab becomes appropriate to
use in second- or third-line therapy, then I might consider it.
Select Publications

