
| Tracks 1-17 |
| Track 1 |
Introduction |
| Track 2 |
Use of bevacizumab after discontinuation of chemotherapy or beyond disease progression |
| Track 3 |
Incorporation of panitumumab into clinical practice |
| Track 4 |
Infusion reactions with cetuximab versus panitumumab |
| Track 5 |
Treatment of potentially curable metastatic disease |
| Track 6 |
Management of synchronous primary and metastatic disease |
| Track 7 |
Patient acceptance of cetuximab-associated rash in trials of adjuvant therapy |
| Track 8 |
Capecitabine alone or with oxaliplatin for the treatment of Stage II disease |
| Track 9 |
Treatment of lower-risk breast and colon cancer |
|
| Track 10 |
Patient acceptance of adjuvant therapy for modest benefits |
| Track 11 |
Patient expectations about chemotherapy side effects |
| Track 12 |
A physician’s experience with a spouse being treated for breast cancer |
| Track 13 |
Impact of a personal experience with cancer on the practice of oncology |
| Track 14 |
Communication and children’s coping with a parent’s cancer |
| Track 15 |
The personal decision to participate in a clinical trial |
| Track 16 |
Impact of cancer diagnosis and treatment on lifestyle and relationships |
| Track 17 |
A personal perspective on the pace of progress in cancer research |
|
|
Select Excerpts from the Interview
Track 2
 DR LOVE:
DR LOVE: What is your general approach to management of metastatic
colorectal cancer in clinical practice?
 DR MARSHALL: I tend to follow an OPTIMOX-type strategy. Whether I’m
administering OPTIMIRI or OPTIMOX, I back off from irinotecan or oxaliplatin
after I see the optimum response, which is usually around four months
of therapy. Generally, I continue with 5-FU and bevacizumab.
DR MARSHALL: I tend to follow an OPTIMOX-type strategy. Whether I’m
administering OPTIMIRI or OPTIMOX, I back off from irinotecan or oxaliplatin
after I see the optimum response, which is usually around four months
of therapy. Generally, I continue with 5-FU and bevacizumab.
The recent OPTIMOX-2 data have given us permission to not administer
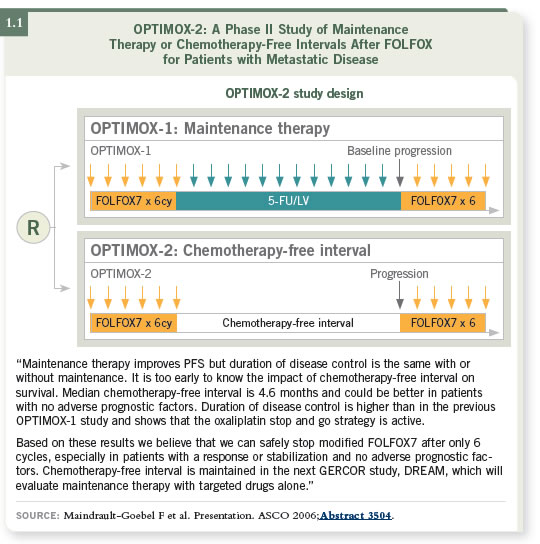
any agent during the chemotherapy-free window, so you could stop treatment altogether (Maindrault-Goebel 2006; [1.1]). However, as a clinical trialist, I
believe that may be the ideal opportunity to bring in new medicines.
We are beginning to design more trials to test new agents in the chemotherapy-free window to see if they are able to stabilize or prolong progression.
When patients reprogress on one regimen, I change to the other base chemotherapy.
However, many physicians like to resume the old chemotherapy:
If the patient was on oxaliplatin, they bring back the oxaliplatin, and if the
patient was on irinotecan, they bring back the irinotecan.
 DR LOVE: When you switch regimens, do you continue the bevacizumab?
DR LOVE: When you switch regimens, do you continue the bevacizumab?
 DR MARSHALL: At that point, I frequently bring in an EGFR blocker, even
though that has not yet been established by the data. More recent trials support
the practice of not waiting until the disease becomes irinotecan refractory
before bringing in an EGFR inhibitor. In fact, data for the EGFR inhibitors
now indicate that in almost every setting they’ve been tested in — last line,
second line, and now we have some first-line data — they’ve shown a positive
impact (Tabernero 2004).
DR MARSHALL: At that point, I frequently bring in an EGFR blocker, even
though that has not yet been established by the data. More recent trials support
the practice of not waiting until the disease becomes irinotecan refractory
before bringing in an EGFR inhibitor. In fact, data for the EGFR inhibitors
now indicate that in almost every setting they’ve been tested in — last line,
second line, and now we have some first-line data — they’ve shown a positive
impact (Tabernero 2004).
 DR LOVE: When you make this decision with the patient whose disease is
progressing, do you factor in how they responded to the treatments?
DR LOVE: When you make this decision with the patient whose disease is
progressing, do you factor in how they responded to the treatments?
 DR MARSHALL: Yes. If they progressed rapidly or didn’t respond well, I’m
less enthusiastic about keeping a drug on board, so I’ll switch it. But if they’ve
received a nice benefit from a drug, I don’t usually give that up.
DR MARSHALL: Yes. If they progressed rapidly or didn’t respond well, I’m
less enthusiastic about keeping a drug on board, so I’ll switch it. But if they’ve
received a nice benefit from a drug, I don’t usually give that up.
With bevacizumab, you can recognize a change in the biology of these tumors
— they slow down. It’s not necessarily that the tumors respond further, it’s just
that you have “turned them off,” so I hesitate to pull patients off of bevacizumab.
Clinically, that’s what we’re seeing — this quieting of colon cancer
and long-term survivors with metastatic disease.
Track 3
 DR LOVE:
DR LOVE: Where does panitumumab currently fit into your treatment
algorithm?
 DR MARSHALL: One of the most common questions I’m getting right now is,
“I’ve used cetuximab. Should I now administer panitumumab?” My answer
has been no.
DR MARSHALL: One of the most common questions I’m getting right now is,
“I’ve used cetuximab. Should I now administer panitumumab?” My answer
has been no.
However, I’m beginning to gain a little more experience with this patient
population, and I administer a lot of BOND-2, last-line regimens (Saltz 2005).
Recognizing that BOND-2 is with anti-EGFR or anti-VEGF treatment-naïve
patients, I will not let patients continue to progress without combining
EGF and VEGF inhibitors at some point. Clinically, when you put the two
antibodies together, you see fairly consistent activity, even in the nonnaïve and
the previously exposed patient.
I have been using cetuximab, and now I have patients who would like to take
the week off. They’re coming to me and saying, “Can I switch off and go to
panitumumab?” and I say, “Sure.” What’s interesting is that when I do so,
I see a little more renewed activity. I’m also seeing a renewed rash. A lot of
patients whose cetuximab rash quieted down are now receiving panitumumab.
Track 5
 DR LOVE:
DR LOVE: Can you discuss your approach to potentially curable
metastatic disease?
 DR MARSHALL: In general, I am increasingly adopting a chemotherapy-first
approach. In my opinion, patients who are going to benefit the most from
a surgical approach — a resection of the metastatic lesion — are those who
respond to chemotherapy (Delaunoit 2005).
DR MARSHALL: In general, I am increasingly adopting a chemotherapy-first
approach. In my opinion, patients who are going to benefit the most from
a surgical approach — a resection of the metastatic lesion — are those who
respond to chemotherapy (Delaunoit 2005).
The marker, if you will, of a responding metastatic lesion is encouraging and
powerful, so more and more patients are being offered surgery for oligo-metastatic
disease. We’re now having an opposite problem — a good problem.
I’m taking care of a young man right now who has a rectal tumor with two
liver metastases and is on a capecitabine trial.
After two rounds of CAPOX and bevacizumab, he has had such a good
response that we can barely see his two liver metastases, and his rectal lesion
has shown a nice response.
If I keep going too much more with chemotherapy, we’re not going to be able
to find the lesions to resect. Not that I think chemotherapy cured the patient,
but the lesions are now not detectable. What a good problem to have. Now we
have to dance that dance and make sure we don’t mismanage the rectal area
and that he still has a shot at a liver resection.
Our decision is that after four cycles we’re going to restage his cancer, then
pause and administer neoadjuvant radiation therapy, maintaining some
capecitabine and probably some oxaliplatin during that radiation therapy. We
will restage after that, and then most likely perform a rectal resection, and at
the same time, instead of a lobectomy, perform radiofrequency ablation on the
residual tumors.
 DR LOVE: What is seen histologically in people who have clinical complete
responses in the liver?
DR LOVE: What is seen histologically in people who have clinical complete
responses in the liver?
 DR MARSHALL: A lot of these people still have residual disease, but some
don’t.
DR MARSHALL: A lot of these people still have residual disease, but some
don’t.
 DR LOVE: How do you decide whether or not to resect the area?
DR LOVE: How do you decide whether or not to resect the area?
 DR MARSHALL: It’s a good question. For this particular patient, I called the
surgeon and asked, “What would you do if you got in there and you couldn’t
see it?” He knows anatomically where the lesions were, so he can resect the
region that they were in. It’s also important to note that even when traditional
imaging shows nothing, an ultrasound can find areas of disease. I was a
little nervous when this patient’s response was so good, thinking that we then
wouldn’t be able to perform what we hoped would be a curative resection on
his liver.
DR MARSHALL: It’s a good question. For this particular patient, I called the
surgeon and asked, “What would you do if you got in there and you couldn’t
see it?” He knows anatomically where the lesions were, so he can resect the
region that they were in. It’s also important to note that even when traditional
imaging shows nothing, an ultrasound can find areas of disease. I was a
little nervous when this patient’s response was so good, thinking that we then
wouldn’t be able to perform what we hoped would be a curative resection on
his liver.
Track 8
 DR LOVE:
DR LOVE: What is your approach to Stage II disease?
 DR MARSHALL: On one side, it’s clear that we’re dramatically overtreating
patients. We’re administering chemotherapy to 100 patients to help what may
be three to six people in the long run. I’m not fundamentally against that, but
I would like to figure out who may benefit and administer chemotherapy to
them. We are trying to recruit to a clinical trial (ECOG-E5202) that groups
patients according to the tumor’s genetic markers, and we’ve had some luck.
DR MARSHALL: On one side, it’s clear that we’re dramatically overtreating
patients. We’re administering chemotherapy to 100 patients to help what may
be three to six people in the long run. I’m not fundamentally against that, but
I would like to figure out who may benefit and administer chemotherapy to
them. We are trying to recruit to a clinical trial (ECOG-E5202) that groups
patients according to the tumor’s genetic markers, and we’ve had some luck.
For the most part, patients are interested in pursuing chemotherapy. Patients
with education — whether it’s fair education or not — will opt to receive
chemotherapy. My feeling is that community physicians are treating more of these people than they were before. They’re also using a lot more capecitabine
in this patient population.
 DR LOVE: So for the patient at lower risk, some physicians are opting for
capecitabine alone because they “want to do something.”
DR LOVE: So for the patient at lower risk, some physicians are opting for
capecitabine alone because they “want to do something.”
 DR MARSHALL: Yes, which doesn’t make sense to me. If you’re going to do
it, do it. The data say that FOLFOX would pick up a couple more people than
capecitabine by itself. I’ve heard Aimery de Gramont say this, and I agree with
him. If I were a Stage II patient, I’d rather receive three months of FOLFOX
than six months of capecitabine alone.
DR MARSHALL: Yes, which doesn’t make sense to me. If you’re going to do
it, do it. The data say that FOLFOX would pick up a couple more people than
capecitabine by itself. I’ve heard Aimery de Gramont say this, and I agree with
him. If I were a Stage II patient, I’d rather receive three months of FOLFOX
than six months of capecitabine alone.
 DR LOVE: What about using capecitabine with oxaliplatin (CAPOX) in the
adjuvant setting?
DR LOVE: What about using capecitabine with oxaliplatin (CAPOX) in the
adjuvant setting?
 DR MARSHALL: The metastatic data with capecitabine and oxaliplatin,
whether it’s infusion or bolus, are positive. So it is probably fine, and then I
put in my little asterisk and say, “But I’ve been wrong before.”
DR MARSHALL: The metastatic data with capecitabine and oxaliplatin,
whether it’s infusion or bolus, are positive. So it is probably fine, and then I
put in my little asterisk and say, “But I’ve been wrong before.”
Select Publications

