
| Tracks 1-19 |
| Track 1 |
Introduction |
| Track 2 |
Case discussion: A woman in her
midfifties presenting with T3N1
rectal cancer |
| Track 3 |
Rationale for preoperative therapy
in the treatment of rectal cancer |
| Track 4 |
Patient’s perception of the need for colorectal screening |
| Track 5 |
Phase II trial of preoperative capecitabine and bevacizumab combined with radiation therapy |
| Track 6 |
Capecitabine versus infusional 5-FU with preoperative radiation therapy |
| Track 7 |
Tolerability and response to neoadjuvant capecitabine/bevacizumab and radiation therapy |
| Track 8 |
Selection of postoperative adjuvant therapy in the treatment of rectal cancer |
| Track 9 |
Incorporating bevacizumab into adjuvant clinical trials |
| Track 10 |
Management of toxicities secondary to adjuvant capecitabine/oxaliplatin |
|
| Track 11 |
Incorporation of bevacizumab
into neoadjuvant clinical trials for
rectal cancer at MD Anderson |
| Track 12 |
Incorporation of oxaliplatin for the
treatment of de novo metastases
or as neoadjuvant therapy for
rectal cancer |
| Track 13 |
Case discussion: A 78-year-old
woman with T3N2M0 colon
cancer and a history of stroke |
| Track 14 |
Comorbidities, performance
status and age as predictors of
tolerability to chemotherapy |
| Track 15 |
Case discussion: A 75-year-old
man with a single focus of hepatic
metastases following resection of
primary colon cancer |
| Track 16 |
Rationale for preoperative
chemotherapy for resectable liver
metastases |
| Track 17 |
Debulking metastatic tumors to
allow for surgical resection |
| Track 19 |
Combining biologic agents in the
treatment of colon cancer |
| Track 19 |
Allergic reactions and the choice
of cetuximab versus panitumumab |
|
|
Select Excerpts from the Interview
Tracks 2-7
 DR LOVE:
DR LOVE: Can you present a case from your practice that exemplifies the
key issues involved with neoadjuvant therapy of rectal cancer?
 DR WOLFF: I recently evaluated a 57-year-old woman who experienced one
or two episodes of rectal bleeding, which didn’t set off any alarms. Then she
had some changes in her bowel habits with more bleeding that prompted her
to see her physician.
DR WOLFF: I recently evaluated a 57-year-old woman who experienced one
or two episodes of rectal bleeding, which didn’t set off any alarms. Then she
had some changes in her bowel habits with more bleeding that prompted her
to see her physician.
A digital rectal examination revealed a mass, and flexible sigmoidoscopy
revealed a mid to low rectal tumor, about five centimeters from the anal verge.
She had T3N1 disease.
 DR LOVE: What treatment options did you discuss with her?
DR LOVE: What treatment options did you discuss with her?
 DR WOLFF: We talked about the rationale for preoperative therapy, such as
improved chances of sphincter preservation. If we tell a patient that we recommend
preoperative therapy and that we have a protocol with a novel molecular
agent — bevacizumab — which has efficacy in advanced disease (Hurwitz
2004) and may have potent radiosensitizing effects (Willett 2005, 2004), the
study is usually of great interest to patients in whom the risks associated with
bevacizumab (myocardial infarction and stroke) are quite low.
DR WOLFF: We talked about the rationale for preoperative therapy, such as
improved chances of sphincter preservation. If we tell a patient that we recommend
preoperative therapy and that we have a protocol with a novel molecular
agent — bevacizumab — which has efficacy in advanced disease (Hurwitz
2004) and may have potent radiosensitizing effects (Willett 2005, 2004), the
study is usually of great interest to patients in whom the risks associated with
bevacizumab (myocardial infarction and stroke) are quite low.
We are currently conducting a Phase II neoadjuvant trial of capecitabine
(administered daily Monday through Friday) and bevacizumab (in weeks one,
three and five) with standard doses of radiation therapy (1.1). We have found
this to be a well-tolerated regimen, and we haven’t seen toxicity above what
we’ve seen with capecitabine and radiation therapy.
 DR LOVE: What is the timing between bevacizumab and surgery?
DR LOVE: What is the timing between bevacizumab and surgery?
 DR WOLFF: We wait at least six weeks between. Patients with rectal cancer
receive chemoradiation therapy followed by six weeks of rest and then a
reevaluation by the surgeon with a proctoscopy.
DR WOLFF: We wait at least six weeks between. Patients with rectal cancer
receive chemoradiation therapy followed by six weeks of rest and then a
reevaluation by the surgeon with a proctoscopy.
 DR LOVE: What did this patient elect to do?
DR LOVE: What did this patient elect to do?
 DR WOLFF: She went on the trial. She experienced what I consider an easy
course of therapy. She had Grade II perianal erythema and some mild moist
desquamation, but she didn’t have severe skin reactions.
DR WOLFF: She went on the trial. She experienced what I consider an easy
course of therapy. She had Grade II perianal erythema and some mild moist
desquamation, but she didn’t have severe skin reactions.
She experienced nice downstaging. Her pathologic stage at surgery was T2N0.
She did not show a complete response, but she was down to microscopic
disease, which makes us feel good about her overall prognosis.
Track 8
 DR LOVE:
DR LOVE: What postoperative recommendation did you provide to this
patient?
 DR WOLFF: I offer patients FOLFOX or CAPOX because I view capecitabine
and infusional 5-FU as essentially equivalent. She opted to take CAPOX
because she had previously received capecitabine.
DR WOLFF: I offer patients FOLFOX or CAPOX because I view capecitabine
and infusional 5-FU as essentially equivalent. She opted to take CAPOX
because she had previously received capecitabine.

Track 12
 DR LOVE:
DR LOVE: The NSABP is conducting the R-04 trial, which is evaluating
capecitabine versus 5-FU with or without oxaliplatin. What are your
thoughts about oxaliplatin in this situation?
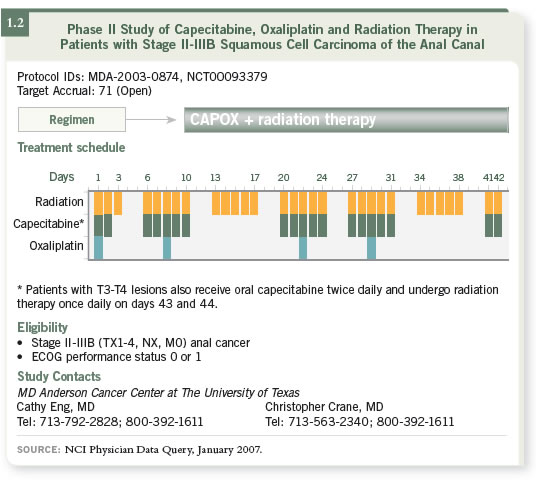
 DR WOLFF: Oxaliplatin is a little more user friendly with radiation therapy
than irinotecan. We are currently conducting a study for patients with anal
cancer evaluating CAPOX combined with radiation therapy (1.2). We are
excited about the results that we are seeing. Every tumor has shown a complete
response, and these responses have been durable. The numbers are small, but
we believe it is a viable strategy.
DR WOLFF: Oxaliplatin is a little more user friendly with radiation therapy
than irinotecan. We are currently conducting a study for patients with anal
cancer evaluating CAPOX combined with radiation therapy (1.2). We are
excited about the results that we are seeing. Every tumor has shown a complete
response, and these responses have been durable. The numbers are small, but
we believe it is a viable strategy.
Tracks 13-14
 DR LOVE:
DR LOVE: Can you discuss your therapeutic approach to older patients
with colon cancer?
 DR WOLFF: I recently saw a 78-year-old woman who had a resected T3N2M0
colon tumor and six positive lymph nodes. She’d had a prior stroke and was
functional, but she needed some assistance from her husband.
DR WOLFF: I recently saw a 78-year-old woman who had a resected T3N2M0
colon tumor and six positive lymph nodes. She’d had a prior stroke and was
functional, but she needed some assistance from her husband.
We wanted to use adjuvant therapy but weren’t comfortable with the idea of
oxaliplatin. We elected to use single-agent capecitabine as adjuvant therapy.
She had a somewhat tough time with some diarrhea, even receiving reduced
doses, but ended up receiving four months of therapy. She is three years out
and doing fine now with no evidence of disease.
 DR LOVE: What were your thoughts on Rich Goldberg’s presentation at ASCO
2006 about the tolerance to chemotherapy in older patients (Sargent 2006)?
DR LOVE: What were your thoughts on Rich Goldberg’s presentation at ASCO
2006 about the tolerance to chemotherapy in older patients (Sargent 2006)?
 DR WOLFF: This is an important research question to ask. From my view, it’s
not so much about age, because I believe the overall take-home message is the
elderly can tolerate this therapy (Sargent 2006).
DR WOLFF: This is an important research question to ask. From my view, it’s
not so much about age, because I believe the overall take-home message is the
elderly can tolerate this therapy (Sargent 2006).
I recently treated a woman who is 72 years old with CAPOX. She came to me
because she had a strong aversion to a two-day infusional pump as part of her
treatment.
Tracks 15-17
 DR WOLFF: Another patient who is relevant to your question about the elderly
is a 75-year-old man who presented with colon cancer and a single focus of
metastatic disease in the periphery of the right lobe of the liver. His primary
tumor had been resected. He was in overall good health with some hypertension.
DR WOLFF: Another patient who is relevant to your question about the elderly
is a 75-year-old man who presented with colon cancer and a single focus of
metastatic disease in the periphery of the right lobe of the liver. His primary
tumor had been resected. He was in overall good health with some hypertension.
His lesion may have been amenable to ablation, which is not typically our
preference if we have the option to resect. Given the choice between ablation
and resection, the data are trending toward resection as always more appropriate.
So he received two or three months of FOLFOX to try to make the tumor
resectable and experienced some nice tumor reduction. He underwent surgery
to remove the hepatic lesion, and it took a little longer than average to recover.
Tracks 18-19
 DR LOVE:
DR LOVE: Do you think the use of chemotherapy with bevacizumab
and cetuximab is rational off protocol in pre-op situations where you are
going for cure?
 DR WOLFF: In select cases it may be. I believe there will be a subset of patients
for whom the biologic doublet, regardless of the chemotherapy backbone, will
provide more bang for your buck. However, I would not be in favor of using
the combination for all patients.
DR WOLFF: In select cases it may be. I believe there will be a subset of patients
for whom the biologic doublet, regardless of the chemotherapy backbone, will
provide more bang for your buck. However, I would not be in favor of using
the combination for all patients.
I usually have a fairly sequential way of going through drugs. If patients start
with FOLFOX/bevacizumab, then they usually receive either FOLFIRI/bevacizumab
or irinotecan as a single agent and then irinotecan and cetuximab.
I tell many of my patients that what they’re trying to accomplish is not a race
— it’s a marathon. You want to stretch out the clock. If you just plow through
your cytotoxics and molecular therapies and put them all into “the soup” at
once, I don’t know what you’re going to have left.
 DR LOVE: In your algorithm, where will panitumumab fit in?
DR LOVE: In your algorithm, where will panitumumab fit in?
 DR WOLFF: Panitumumab will probably be used with regimens like FOLFIRI
on an every two-week schedule. It will be more convenient than receiving
weekly cetuximab. Furthermore, physicians will be excited by the fact that
allergic reactions aren’t common with panitumumab.
DR WOLFF: Panitumumab will probably be used with regimens like FOLFIRI
on an every two-week schedule. It will be more convenient than receiving
weekly cetuximab. Furthermore, physicians will be excited by the fact that
allergic reactions aren’t common with panitumumab.
Select Publications

