
| Tracks 1-16 |
| Track 1 |
Introduction |
| Track 2 |
Development of cetuximab and panitumumab |
| Track 3 |
Potential mechanisms of action of cetuximab and panitumumab |
| Track 4 |
Cetuximab-associated infusion reactions |
| Track 5 |
Relationship between infusion reactions and geographic variables |
| Track 6 |
Predictors of response to EGFR inhibitors |
| Track 7 |
Relationship between serum LDH and benefit from VEGF inhibitors |
| Track 8 |
Clinical management of metastatic colon cancer in the first-line setting |
|
| Track 9 |
Cetuximab-associated skin toxicity |
| Track 10 |
Clinical management of cetuximab-associated skin toxicity |
| Track 11 |
Considerations in evaluating bevacizumab and EGFR inhibitors in the adjuvant setting |
| Track 12 |
Assessment of EGFR |
| Track 13 |
Geographic variability in the side effects of fluoropyrimidines |
| Track 14 |
Relationship between folic acid and the side effects of fluoropyrimidines |
| Track 15 |
Mechanism of fluoropyrimidine toxicity |
| Track 16 |
Impact of diet and exercise on colorectal cancer |
|
|
Select Excerpts from the Interview
Track 3
 DR LOVE:
DR LOVE: Can you discuss what we know about the mechanism of action
of cetuximab and panitumumab?
 DR LENZ: These are two monoclonal antibodies that both inhibit the
epidermal growth factor receptor (EGFR). The EGFR is a critical mainstay of
tumor development, tumor progression, metastasis and invasion.
DR LENZ: These are two monoclonal antibodies that both inhibit the
epidermal growth factor receptor (EGFR). The EGFR is a critical mainstay of
tumor development, tumor progression, metastasis and invasion.
When you examine the data for either one of these two agents, you see
efficacy in the third- and fourth-line settings. This provides clues that the
EGFR is untouched by classical chemotherapy — there are no mechanisms of
cross resistance — and shows how important this receptor is in the process of
tumor progression.
We want to understand which patients might benefit most from these therapies.
The first goal is to evaluate the mechanism of resistance of cetuximab.
We went back to the literature and found data from animal models showing
that when tumors overexpress vascular endothelial growth factor (VEGF),
cetuximab does not work.
In our clinical trial at the University of Southern California, 40 patients were
treated with cetuximab, again in the third- and fourth-line settings. When
we measured VEGF in the tumor, that’s exactly what we found: Tumors with
high levels of VEGF do not respond to cetuximab (Vallböhmer 2005).
 DR LOVE: Is the VEGF receptor found on the tumor cells?
DR LOVE: Is the VEGF receptor found on the tumor cells?
 DR LENZ: Yes. The VEGF receptor is expressed not only on the endothelial
cells but also significantly on tumor cells (Fan 2005). It is interesting because
with anti-VEGF treatment you have an anti-angiogenic effect as well as an
antitumor effect.
DR LENZ: Yes. The VEGF receptor is expressed not only on the endothelial
cells but also significantly on tumor cells (Fan 2005). It is interesting because
with anti-VEGF treatment you have an anti-angiogenic effect as well as an
antitumor effect.
Track 8
 DR LOVE:
DR LOVE: In general, how do you approach first-line therapy in
metastatic colon cancer?
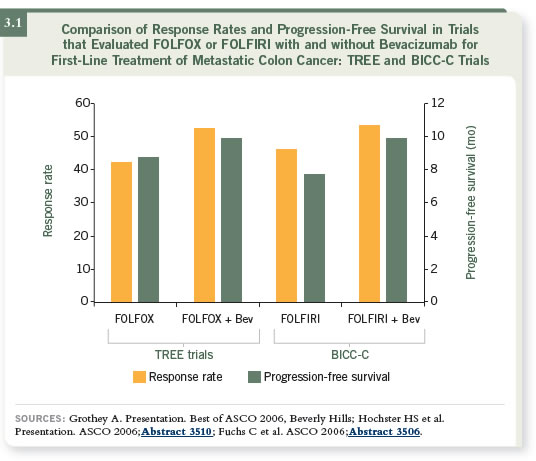
 DR LENZ: I usually use either a backbone of FOLFOX or FOLFIRI, usually
combined with bevacizumab. We know some patients will benefit more
from FOLFIRI and others will benefit more from FOLFOX, and that will
also be true for bevacizumab and cetuximab (3.1). We know that bevacizumab
has little activity as monotherapy. It needs chemotherapy to be effective
cytotoxically.
DR LENZ: I usually use either a backbone of FOLFOX or FOLFIRI, usually
combined with bevacizumab. We know some patients will benefit more
from FOLFIRI and others will benefit more from FOLFOX, and that will
also be true for bevacizumab and cetuximab (3.1). We know that bevacizumab
has little activity as monotherapy. It needs chemotherapy to be effective
cytotoxically.
Track 14
 DR LOVE:
DR LOVE: What did you think about the presentation done at ASCO
2006 evaluating the side effects of fluoropyrimidine monotherapy based
on geography (3.2)?
 DR LENZ: Dan Haller presented these data, and he comprehensively evaluated
the frequency of side effects of 5-FU or capecitabine in the United States
and the rest of the world (Haller 2006).
DR LENZ: Dan Haller presented these data, and he comprehensively evaluated
the frequency of side effects of 5-FU or capecitabine in the United States
and the rest of the world (Haller 2006).
An ongoing discrepancy exists between the toxicities reported in Europe and
the United States, and we know there are significant differences in 5-FU
toxicity among different ethnic populations. Asians, African-Americans and
Caucasians experience different levels of toxicity. That is explained by the
genetic make-up of the patient — not the tumor.
The biggest difference between Europe and the United States is the supplementation
of food with folates, which has a significant benefit for cardiovascular
and neurological development and so on. In Europe, folate supplementation
is not common. We also know that Americans are much more eager to
supplement their diet with vitamins, including folic acid.
We believe one of the major explanations for the differences in f luoropyrimidine
toxicities may be the supplement of folate in our food and the intake of vitamin
supplements. The more supplementation of folate, the higher the toxicity.
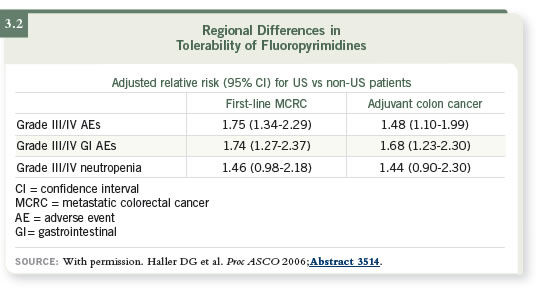
We also believe another reason may be some difference of genetic background,
because our populations have changed and the genetic pool is not as homogeneous
as when the immigrants came over from Europe.
However, I don’t believe that’s the only explanation. I believe there is a
lifestyle factor in that equation. From my point of view, the most reasonable
explanation for the differences in toxicities by region is a combination of
genetic background and folate supplementation, and that’s exactly what Dan
Haller concluded.
Select Publications

