
| Tracks 1-23 |
| Track 1 |
Introduction |
| Track 2 |
Comparison of the EGFR inhibitors cetuximab and panitumumab in colon cancer |
| Track 3 |
Clinical trials with panitumumab |
| Track 4 |
Therapeutic algorithm for metastatic colon cancer |
| Track 5 |
Cetuximab-associated infusion reactions |
| Track 6 |
Incidence of infusion reactions for cetuximab versus panitumumab |
| Track 7 |
Combination therapy with an EGFR antibody and bevacizumab |
| Track 8 |
Chemotherapy plus double
biologics for potentially curable
hepatic metastases |
| Track 9 |
Predictors of response to EGFR inhibitors |
| Track 10 |
Novel agents and strategies to inhibit multiple pathways |
| Track 11 |
Clinical trials with multitargeted TKIs |
| Track 12 |
Potential mechanisms of action of bevacizumab |
|
| Track 13 |
Bevacizumab-associated side effects and use in the adjuvant setting |
| Track 14 |
Utilization of bevacizumab for elderly patients |
| Track 15 |
Implications of bevacizumab-associated hypertension |
| Track 16 |
Treatment of bevacizumab-associated hypertension |
| Track 17 |
Phase I trials of insulin-like growth factor receptor antagonists |
| Track 19 |
Potential impact of lifestyle modifications on risk of cancer recurrence |
| Track 19 |
Ongoing studies in the adjuvant and neoadjuvant settings |
| Track 20 |
Phase II study evaluating cetuximab with erlotinib |
| Track 21 |
Targeted therapy combining TKIs and antibody therapy |
| Track 22 |
Patients’ acceptance of serial biopsies in the clinical trial setting |
| Track 23 |
Rash secondary to combined TKI and antibody therapy |
|
|
Select Excerpts from the Interview
Track 3
 DR LOVE:
DR LOVE: Can you review what has been seen in clinical trials with
panitumumab?
 DR BERLIN: This agent has been tested in metastatic disease in a randomized
trial versus best supportive care (Peeters 2006) and as first-line therapy in
combination with irinotecan-containing regimens (Hecht 2006).
DR BERLIN: This agent has been tested in metastatic disease in a randomized
trial versus best supportive care (Peeters 2006) and as first-line therapy in
combination with irinotecan-containing regimens (Hecht 2006).
Progression-free survival was well over 10 months, which corresponds with
what we have seen thus far with the newer bevacizumab-containing regimens.
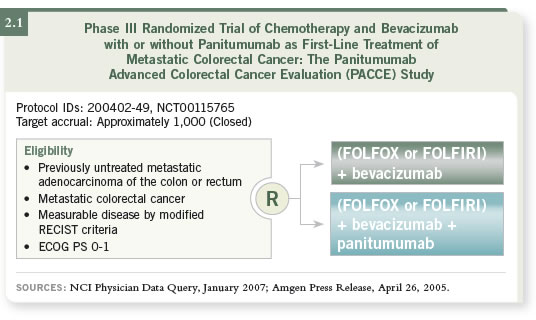
The PACCE (Panitumumab Advanced Colorectal Cancer Evaluation)
trial, which has completed accrual, is evaluating panitumumab with either
FOLFIRI or FOLFOX plus bevacizumab (2.1). Those data, at least for
toxicity, should be available soon.
The current availability of panitumumab is based on data from the Phase
III trial in which patients were randomly assigned to panitumumab or best
supportive care in the third-line setting (Peeters 2006; [2.2]).
Crossover was allowed, meaning that patients initially assigned to best
supportive care were able to go on to panitumumab as soon as their doctors
considered that their disease had progressed. That may have played a role in
the results, but the bottom line was that the panitumumab group had a better
progression-free survival than the best supportive care group.
No survival difference appeared, but a large majority of the patients on the
best supportive care arm actually received panitumumab, so we assume that
this played a role in the lack of survival benefit.
Tracks 4-6
 DR LOVE:
DR LOVE: What is your clinical algorithm for the treatment of metastatic
colon cancer?
 DR BERLIN: We start with bevacizumab in combination with either FOLFIRI
or FOLFOX first line. Because of the clinical trials we have participated in,
we tend to start more often with FOLFIRI than with FOLFOX. We then
switch to the other regimen in the second line — if we start with FOLFIRI,
we switch to FOLFOX.
DR BERLIN: We start with bevacizumab in combination with either FOLFIRI
or FOLFOX first line. Because of the clinical trials we have participated in,
we tend to start more often with FOLFIRI than with FOLFOX. We then
switch to the other regimen in the second line — if we start with FOLFIRI,
we switch to FOLFOX.

We do not continue bevacizumab beyond the first-line setting, and we use
irinotecan with cetuximab as third-line therapy. However, that may change to
irinotecan in combination with panitumumab.
 DR LOVE: What are your thoughts on the issue of every two-week scheduling
of panitumumab and infusion reactions?
DR LOVE: What are your thoughts on the issue of every two-week scheduling
of panitumumab and infusion reactions?
 DR BERLIN: Every two-week scheduling reduces cost to some extent because
you’re not paying the infusion cost every week. We also are interested in every
two-week scheduling to minimize infusion reactions because we are in an area
where the cetuximab-associated infusion reaction is more common.
DR BERLIN: Every two-week scheduling reduces cost to some extent because
you’re not paying the infusion cost every week. We also are interested in every
two-week scheduling to minimize infusion reactions because we are in an area
where the cetuximab-associated infusion reaction is more common.
 DR LOVE: Rich Goldberg from North Carolina has also talked about the high
incidence of cetuximab-associated infusion reaction. Do you believe there is a
regional relationship to infusion reactions?
DR LOVE: Rich Goldberg from North Carolina has also talked about the high
incidence of cetuximab-associated infusion reaction. Do you believe there is a
regional relationship to infusion reactions?
 DR BERLIN: We believe it’s real. We do not believe it’s a statistical fluke,
because of the volume of patients we’ve treated and the volume of patients
treated elsewhere. In addition, we have a physician who transferred from New
Orleans, who had worked with cetuximab in head and neck cancer for over
a year, had never seen an infusion reaction and has yet to administer cetuximab
without an infusion reaction at Vanderbilt. We are running around a 15
percent Grade III or Grade IV infusion reaction rate.
DR BERLIN: We believe it’s real. We do not believe it’s a statistical fluke,
because of the volume of patients we’ve treated and the volume of patients
treated elsewhere. In addition, we have a physician who transferred from New
Orleans, who had worked with cetuximab in head and neck cancer for over
a year, had never seen an infusion reaction and has yet to administer cetuximab
without an infusion reaction at Vanderbilt. We are running around a 15
percent Grade III or Grade IV infusion reaction rate.
 DR LOVE: What other geographic areas are seeing a high incidence of cetuximab-associated infusion reactions?
DR LOVE: What other geographic areas are seeing a high incidence of cetuximab-associated infusion reactions?
 DR BERLIN: The regions that report high rates of infusion reactions with
cetuximab appear to be some areas of North Carolina, South Carolina and
Tennessee. It is not seen as much in the higher elevations of these regions.
Whether it’s the higher elevation or the specific location is not clear, but it is
not seen as much there. We are currently working on a paper on this subject
that includes patients from Vanderbilt, the University of North Carolina and
the Sarah Cannon Cancer Center, which is a large network of cancer centers.
DR BERLIN: The regions that report high rates of infusion reactions with
cetuximab appear to be some areas of North Carolina, South Carolina and
Tennessee. It is not seen as much in the higher elevations of these regions.
Whether it’s the higher elevation or the specific location is not clear, but it is
not seen as much there. We are currently working on a paper on this subject
that includes patients from Vanderbilt, the University of North Carolina and
the Sarah Cannon Cancer Center, which is a large network of cancer centers.
 DR LOVE: Could this phenomenon be related to the pharmacogenetics of the
people in certain areas, or is it environmental?
DR LOVE: Could this phenomenon be related to the pharmacogenetics of the
people in certain areas, or is it environmental?
 DR BERLIN: In modern day America it is more likely related to environment,
because our patients don’t come from just one area. They are originally from
different areas — they are not just people who are native to Tennessee. We see
a variety of patients from all over the world.
DR BERLIN: In modern day America it is more likely related to environment,
because our patients don’t come from just one area. They are originally from
different areas — they are not just people who are native to Tennessee. We see
a variety of patients from all over the world.
 DR LOVE: What exactly do we know about the incidence of infusion reactions
with panitumumab?
DR LOVE: What exactly do we know about the incidence of infusion reactions
with panitumumab?
 DR BERLIN: I have yet to hear about a patient who has had an infusion
reaction with panitumumab. The infusion reaction rate is less than one percent,
and the patients who experience a panitumumab-associated infusion reaction
are generally able to receive the drug a second time with premedication.
DR BERLIN: I have yet to hear about a patient who has had an infusion
reaction with panitumumab. The infusion reaction rate is less than one percent,
and the patients who experience a panitumumab-associated infusion reaction
are generally able to receive the drug a second time with premedication.
Track 7
 DR LOVE:
DR LOVE: Can you comment on the combination of an EGFR antibody
and bevacizumab?
 DR BERLIN: At this point, we don’t know if that’s truly beneficial. A couple of
years ago when Dr Saltz presented data on the BOND-2 trial, he showed that
the combination of cetuximab and bevacizumab looked better than cetuximab
alone from the BOND-1 trial, and the combination of irinotecan/cetuximab/
bevacizumab looked better than irinotecan/cetuximab alone (Saltz 2005).
DR BERLIN: At this point, we don’t know if that’s truly beneficial. A couple of
years ago when Dr Saltz presented data on the BOND-2 trial, he showed that
the combination of cetuximab and bevacizumab looked better than cetuximab
alone from the BOND-1 trial, and the combination of irinotecan/cetuximab/
bevacizumab looked better than irinotecan/cetuximab alone (Saltz 2005).
Of course, comparing trial to trial is problematic. BOND-2 was a small, Phase
II trial. So we don’t know if the differences are real, but they provide a good
rationale for what has been called “horizontal targeting,” or targeting two
separate pathways.
The PACCE trial (2.1) and the Intergroup trial (C80405) investigated whether
double-antibody therapy in the first-line setting is better. The Intergroup
trial is evaluating chemotherapy (FOLFIRI or FOLFOX) with cetuximab or
bevacizumab or both.
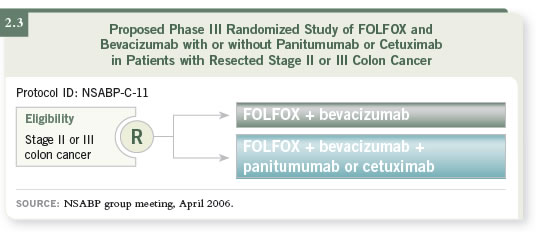
 DR LOVE: The NSABP is considering adding an EGFR inhibitor to
FOLFOX/bevacizumab for their next adjuvant trial (2.3). What do you think
about that strategy?
DR LOVE: The NSABP is considering adding an EGFR inhibitor to
FOLFOX/bevacizumab for their next adjuvant trial (2.3). What do you think
about that strategy?
 DR BERLIN: I believe that is a reasonable leap, and I am much in favor of it. I
don’t know which EGFR inhibitor the NSABP will settle on, but I know that
they will use one of the antibodies in combination with FOLFOX/bevacizumab
versus FOLFOX/bevacizumab alone.
DR BERLIN: I believe that is a reasonable leap, and I am much in favor of it. I
don’t know which EGFR inhibitor the NSABP will settle on, but I know that
they will use one of the antibodies in combination with FOLFOX/bevacizumab
versus FOLFOX/bevacizumab alone.
Track 14
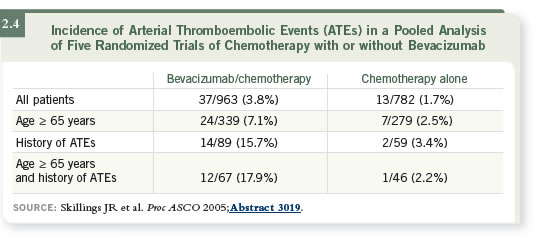
 DR LOVE: What is your approach to using bevacizumab in patients with
prior arterial events?
DR LOVE: What is your approach to using bevacizumab in patients with
prior arterial events? DR BERLIN: Patients who were 65 years of age and older with a prior event
had more than a 17 percent risk of a second event while on bevacizumab
— quite a substantial risk (2.4). However, we have a number of 65-year-old
patients who have had a previous MI and are receiving bevacizumab. We have
warned them about the potential for arterial events, but it’s hard not to recommend
a drug with a survival benefit this good.
DR BERLIN: Patients who were 65 years of age and older with a prior event
had more than a 17 percent risk of a second event while on bevacizumab
— quite a substantial risk (2.4). However, we have a number of 65-year-old
patients who have had a previous MI and are receiving bevacizumab. We have
warned them about the potential for arterial events, but it’s hard not to recommend
a drug with a survival benefit this good.
Track 16
 DR LOVE:
DR LOVE: How do you approach treatment of hypertension associated
with bevacizumab in a patient with metastatic disease?
 DR BERLIN: We tend to use the beta blockers or the ACE inhibitors. We
treat patients on bevacizumab more aggressively for hypertension because of
the potential for reversible posterior leukoencephalopathy syndrome (RPLS),
which can be mistaken for a stroke or a TIA. The syndrome can include
confusion, symptoms of a stroke, seizures or even coma or death. RPLS happens rarely, but almost always in conjunction with at least some level of
hypertension, and treating the hypertension usually leads to reversibility.
DR BERLIN: We tend to use the beta blockers or the ACE inhibitors. We
treat patients on bevacizumab more aggressively for hypertension because of
the potential for reversible posterior leukoencephalopathy syndrome (RPLS),
which can be mistaken for a stroke or a TIA. The syndrome can include
confusion, symptoms of a stroke, seizures or even coma or death. RPLS happens rarely, but almost always in conjunction with at least some level of
hypertension, and treating the hypertension usually leads to reversibility.
Select Publications

