You are here: Home: CCU 2 | 2006: Joel Tepper, MD

Tracks 1-16 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Increased incidence of rectal
cancer in men treated with
radiation therapy for prostate
cancer |
| Track 3 |
Impact of T stage and N stage
on survival of patients with
rectal cancer |
| Track 4 |
Impact of the number of retrieved
nodes on outcome in patients
with rectal cancer |
| Track 5 |
Adjuvant chemotherapy following
neoadjuvant chemoradiation
therapy for rectal cancer |
| Track 6 |
Preoperative evaluation of
patients with rectal cancer |
| Track 7 |
Indications for sphincter-sparing
surgical procedures in rectal
cancer |
| Track 8 |
Impact of surgeon experience on
outcome in rectal cancer |
|
| Track 9 |
NSABP neoadjuvant and ECOG
adjuvant rectal cancer trials |
| Track 10 |
Infusional 5-FU versus
capecitabine in combination
with radiation therapy for
rectal cancer |
| Track 11 |
Oxaliplatin in combination with
preoperative radiation therapy for
rectal cancer |
| Track 12 |
Potential role of bevacizumab in
the management of rectal cancer |
| Track 13 |
Local excision and radiation
therapy for the management of
rectal cancer |
| Track 14 |
Use of intraoperative radiation
therapy for patients with locally
advanced rectal cancer |
| Track 15 |
Importance of tailoring radiation
fields to the individual patient’s
anal and rectal anatomy |
| Track 16 |
Future directions in the
management of colon and
rectal cancers |
|
|
Select Excerpts from the Interview
 Track 3-4 Track 3-4
 DR LOVE: What have we learned over the past couple of years about
staging rectal cancer? DR LOVE: What have we learned over the past couple of years about
staging rectal cancer? |
 DR TEPPER: First of all, I want to emphasize that we are in an age driven by
molecular biology and elaborate ways of evaluating disease. However, what we
have found is that some very standard, straightforward factors can make a real
difference in terms of understanding the risks and outcomes of the disease. We need to emphasize the importance of what goes on both surgically and pathologically
with the disease and how we interpret these data. DR TEPPER: First of all, I want to emphasize that we are in an age driven by
molecular biology and elaborate ways of evaluating disease. However, what we
have found is that some very standard, straightforward factors can make a real
difference in terms of understanding the risks and outcomes of the disease. We need to emphasize the importance of what goes on both surgically and pathologically
with the disease and how we interpret these data.
A series of articles has been published that has taken the lead in examining
the impact of T stage and N stage on outcome for patients with rectal cancer
(Gunderson 2002, 2004). It’s been known for years that N stage is important.
In fact, many treatment algorithms for rectal cancer are based almost entirely
on the premise that N stage dominates everything in terms of outcome.
However, the data from these studies show that both the T stage and the N
stage of the tumor are important, and the impact of each on disease outcome
is somewhat independent of the other. If you look at the data carefully, it
is evident that someone with T2/N1 disease might have a slightly better
prognosis than someone who has T3/N0 disease.
In a patient with N0 disease or, perhaps, N1 disease, the T stage is significant
in determining the risk for both local and distant recurrence. So T stage has a
big impact on survival differences, far larger than any impact that results from
a therapeutic intervention additive to surgery, such as chemotherapy, radiation
therapy, or a combination of the two.
In a study published several years ago, we evaluated tissue from patients with
rectal cancer in terms of T and N staging (Tepper 2001). The study was based
on an analysis of patients from the Gastrointestinal (GI) Intergroup study
0114, in which all patients with rectal cancer were treated postoperatively with
chemotherapy and radiation/chemotherapy and then more chemotherapy. No
difference appeared between the therapeutic interventions, so we were able to
combine all of the treatment categories together.
When we looked at the data, we found that the number of lymph nodes
examined had a great impact on outcome. More specifically, among patients
with T3/N0 disease, the outcome was far worse if the pathologist found zero
to eight nodes compared to finding 14 or more nodes in the specimen. Again,
this difference was much bigger than the differences we see among therapeutic
interventions.
This suggests to me the extreme importance of how we select patients who are
at high risk, both in terms of how we define the outcomes of studies as well
as how we define whom we should and shouldn’t treat with adjuvant therapies.
For example, it is possible that a patient with T3/N0 disease, which has
been well staged with many nodes that are negative, may not need adjuvant
therapy, or at least not the same level of adjuvant therapy as he or she would
need otherwise.
Another issue is whether nodal pathology outcomes are related to the
surgeon’s skill and how well the operation was performed or to how well and
how carefully the pathologist examined the specimen. We can’t answer this
question definitively from this study, but the data suggest that the pathologist
had a greater effect on outcomes than the surgeon. This is based on the fact
that the number of nodes the pathologist found appeared to have a large effect
on outcome for the patients with T3/N0 disease.
For patients with T3/N1 disease, there was a lesser effect, and for patients with
T3/N2 disease, no discernible effect was visible. This suggests stage migration.
If the effect was based simply on the surgeon performing a better operation,
I would expect that the largest effect would be among the patients with N2
disease.
In reality, good surgery and good pathology probably both count, not just one
or the other.
 DR LOVE: What about the prognosis for patients who have received neoadjuvant
radiation therapy and chemotherapy for rectal cancer? How do you interpret
nodes? DR LOVE: What about the prognosis for patients who have received neoadjuvant
radiation therapy and chemotherapy for rectal cancer? How do you interpret
nodes?
 DR TEPPER: It’s harder to interpret the node count for those patients. One
still should be able to define nodes in those patients, but the gold standard of
a minimum of 13 or 14 nodes probably doesn’t apply, and you probably can’t
have that as a reasonable standard. DR TEPPER: It’s harder to interpret the node count for those patients. One
still should be able to define nodes in those patients, but the gold standard of
a minimum of 13 or 14 nodes probably doesn’t apply, and you probably can’t
have that as a reasonable standard.
A couple of studies have examined this issue and found an increased difficulty
in finding nodes in those patients (Wichmann 2002; Beresford 2005; Thorn
2004; Luna-Perez 2003).
 Track 9 Track 9
 DR LOVE: What new clinical research approaches are currently under way
for rectal cancer, including combined chemotherapy/radiation therapy
regimens? DR LOVE: What new clinical research approaches are currently under way
for rectal cancer, including combined chemotherapy/radiation therapy
regimens? |
 DR TEPPER: We’ve tried to coordinate the adjuvant trials in rectal cancer
in the United States through a group previously called the GI Intergroup,
which is now known as the GI Steering Committee of the National Cancer
Institute’s Clinical Trials Working Group. DR TEPPER: We’ve tried to coordinate the adjuvant trials in rectal cancer
in the United States through a group previously called the GI Intergroup,
which is now known as the GI Steering Committee of the National Cancer
Institute’s Clinical Trials Working Group.
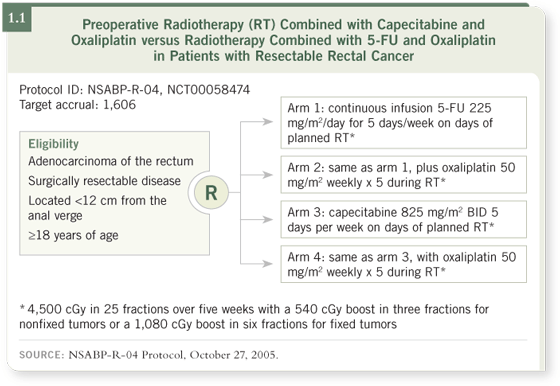
Two carefully designed trials have recently started that allow patients to enroll
in both studies. The first is the NSABP-R-04 study that is evaluating preoperative
radiation and chemotherapy for rectal cancer patients.
The study has a two-by-two randomization scheme in which patients are
initially assigned either to continuous infusion 5-FU or to capecitabine plus or
minus oxaliplatin, with radiation therapy.
The idea was to use oxaliplatin to increase the rate of pathologic complete
response, improve the local control rate, and perhaps offer additional beneficial
systemic effects (1.1).
After patients complete preoperative therapy and undergo surgery, they can
be enrolled in an ECOG study in which patients are randomly assigned to
FOLFOX either alone or with bevacizumab.
The entry criteria are basically what they’ve been for almost all of the rectal
studies to date: The patients must have T3 and/or node-positive disease.
Select publications
|

