You are here: Home: CCU 2 | 2006: Josep Tabernero, MD

Tracks 1-14 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Background to the AVANT
adjuvant trial: Safety and efficacy
of CAPOX with bevacizumab |
| Track 3 |
Arteriovascular exclusionary
criteria in the AVANT trial |
| Track 4 |
Adjuvant chemotherapy for
patients with Stage II and III
disease |
| Track 5 |
Clinical implications of the X-ACT
adjuvant trial results |
| Track 6 |
Capecitabine dosing in the
adjuvant setting |
| Track 7 |
Proposed trials of cetuximab in
the adjuvant setting |
| Track 8 |
Incorporating bevacizumab/
cetuximab therapies into
clinical practice |
|
| Track 9 |
Clinical trial results of irinotecan-containing
regimens in the
adjuvant setting |
| Track 10 |
NSABP-C-07: Comparison
of toxicity with FLOX versus
FOLFOX |
| Track 11 |
Role of calcium and magnesium
for oxaliplatin-related neurotoxicity
in the adjuvant and
metastatic settings |
| Track 12 |
Management of oxaliplatin-related
neuropathy |
| Track 13 |
Safety and efficacy of
neoadjuvant oxaliplatin for
rectal cancer |
| Track 14 |
Future directions in the
management of colorectal
cancer |
|
|
Select Excerpts from the Interview
 Track 2 Track 2
 DR LOVE: What was the rationale for looking at CAPOX in the AVANT
trial? DR LOVE: What was the rationale for looking at CAPOX in the AVANT
trial? |
 DR TABERNERO: Results from a large Phase II study in metastatic disease
showed that CAPOX (oxaliplatin/capecitabine) has at least the same efficacy as
FOLFOX-4 (Tabernero 2002), so the next step was to further test this combination
in both the metastatic and adjuvant settings. DR TABERNERO: Results from a large Phase II study in metastatic disease
showed that CAPOX (oxaliplatin/capecitabine) has at least the same efficacy as
FOLFOX-4 (Tabernero 2002), so the next step was to further test this combination
in both the metastatic and adjuvant settings.
In the metastatic setting, preliminary results of Phase III studies show that
CAPOX has the same efficacy in terms of response rate and time to progression
and a better safety profile than oxaliplatin and 5-fluorouracil-based
combinations (Sastre 2005; Arkenau 2005).
So we want to move this chemotherapy schedule to the adjuvant setting. In
addition to safety, another important issue with this schedule is patient convenience.
With CAPOX patients come to the hospital or medical facility once
every three weeks, and they take pills for two weeks at home.
 DR LOVE: What do we know about the combination of CAPOX and bevacizumab? DR LOVE: What do we know about the combination of CAPOX and bevacizumab?
 DR TABERNERO: We have the results of two different studies. The first study
was the TREE-2 study (Hochster 2005a, 2006), which was a randomized
Phase II study with three different arms. One of the arms studied a combination
of CAPOX and bevacizumab. Data from this arm were compared with
those from an arm of the TREE-1 study that studied CAPOX alone. DR TABERNERO: We have the results of two different studies. The first study
was the TREE-2 study (Hochster 2005a, 2006), which was a randomized
Phase II study with three different arms. One of the arms studied a combination
of CAPOX and bevacizumab. Data from this arm were compared with
those from an arm of the TREE-1 study that studied CAPOX alone.
These data showed a clear increase in response rate with a similar safety profile
compared to CAPOX alone (Hochster 2005b).
The other experience we have right now is that presented by Dr Fernando this
year at the ASCO meeting (Fernando 2005). It was interesting to see that the
median time to progression was almost 12 months using a different schedule of
CAPOX and bevacizumab. That is very relevant.
 DR LOVE: Do you think the combination of CAPOX and bevacizumab is a
rational first-line clinical alternative for metastatic disease? DR LOVE: Do you think the combination of CAPOX and bevacizumab is a
rational first-line clinical alternative for metastatic disease?
 DR TABERNERO: I would say yes. From a regulatory point of view, you
definitely need the data from Phase III studies, but at this time, especially in
the United States, some physicians are treating patients with the combination
of CAPOX and bevacizumab. DR TABERNERO: I would say yes. From a regulatory point of view, you
definitely need the data from Phase III studies, but at this time, especially in
the United States, some physicians are treating patients with the combination
of CAPOX and bevacizumab.
The safety reports from patients who are treated with this regimen do not
cause us to anticipate any different safety issues than those associated with
FOLFOX-4.
 Track 3 Track 3
 DR LOVE: What are the exclusion criteria for the AVANT trial in terms
of prior cardiovascular disease? DR LOVE: What are the exclusion criteria for the AVANT trial in terms
of prior cardiovascular disease? |
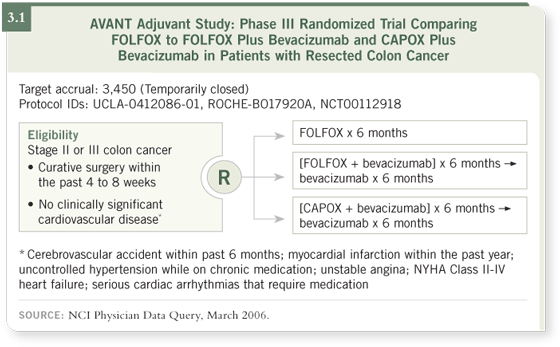
 DR TABERNERO: They rule out patients with what we call active ischemic
arterial disease — not only cardiac disease, but also other arterial diseases (3.1).
This means that patients who’ve had an arterial event must be without signs
and symptoms for one year with disease control. DR TABERNERO: They rule out patients with what we call active ischemic
arterial disease — not only cardiac disease, but also other arterial diseases (3.1).
This means that patients who’ve had an arterial event must be without signs
and symptoms for one year with disease control.
The same goes for arterial hypertension. Arterial hypertension must be well
controlled with either diet or medical treatment.
But this is only one requirement for treating patients in the AVANT study.
The other thing we stress to the patients is that they need close follow-up. I
request at least three blood pressure readings per week for the first treatment
cycle.
If I see that the patient has no problem with hypertension, I reduce the
follow-up requirement. But at the beginning of treatment, I think this is
important.
 Track 4 Track 4
 DR LOVE: Can you talk about your decision-making approach off
protocol in terms of adjuvant therapy for Stage III and Stage II disease? DR LOVE: Can you talk about your decision-making approach off
protocol in terms of adjuvant therapy for Stage III and Stage II disease? |
 DR TABERNERO: I’m convinced of the effects of oxaliplatin-based chemotherapy,
especially FOLFOX-4, in the adjuvant setting. DR TABERNERO: I’m convinced of the effects of oxaliplatin-based chemotherapy,
especially FOLFOX-4, in the adjuvant setting.
I discuss the risks of disease recurrence with patients with a 5-fluorouracil/leucovorin-based chemotherapy or FOLFOX. Very few patients are opposed
to treatment with oxaliplatin-based chemotherapy, so my standard has been to
begin patients with FOLFOX-4 for 12 cycles.
 DR LOVE: How do you approach Stage II disease? DR LOVE: How do you approach Stage II disease?
 DR TABERNERO: I give the same recommendations; although the recurrence
risk is lower for some patients with Stage II disease, for others it’s even higher
than that for Stage III disease. I believe the relative reduction in the recurrence
risk is almost the same with chemotherapy. DR TABERNERO: I give the same recommendations; although the recurrence
risk is lower for some patients with Stage II disease, for others it’s even higher
than that for Stage III disease. I believe the relative reduction in the recurrence
risk is almost the same with chemotherapy.
Chemotherapy does not discriminate the tumor stage, and chemotherapy
reduces the risk of relapse. You need to determine not only the absolute
figures but also the relative figures.
Patients who are in very good condition, in a very healthy state, without any
disease that might compromise their life in the next five to seven years usually
want to have as much treatment as possible to decrease the risk of recurrence.
So my recommendation is to give FOLFOX-4 to patients with high-risk Stage
II disease.
 Track 5 Track 5
 DR LOVE: Could you summarize your take on the X-ACT trial? DR LOVE: Could you summarize your take on the X-ACT trial? |
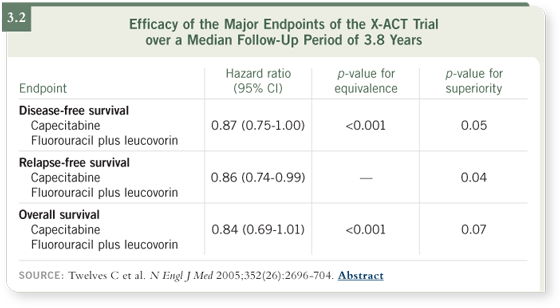
 DR TABERNERO: When the X-ACT trial (Twelves 2005) results were
presented, they impressed us because we had expected equivalence between
capecitabine and 5-fluorouracil/leucovorin. Instead, we were shown some
impressive advantages in relapse-free survival and disease-free survival (3.2). DR TABERNERO: When the X-ACT trial (Twelves 2005) results were
presented, they impressed us because we had expected equivalence between
capecitabine and 5-fluorouracil/leucovorin. Instead, we were shown some
impressive advantages in relapse-free survival and disease-free survival (3.2).
I think the good news is that capecitabine is an alternative for patients who are
reluctant to receive oxaliplatin because they fear polyneuropathy or they may
even fear receiving intravenous chemotherapy.
The only bad news from the X-ACT study was that patients with high-risk
Stage II disease were not included in the trial.
So, unfortunately, the regulatory approval of capecitabine will include only
patients with Stage III disease. In some countries, it might be difficult at times
to use capecitabine in the adjuvant treatment of patients with high-risk Stage
II disease.
 Track 13 Track 13
 DR LOVE: In a clinical setting, how do you decide between capecitabine
and infusional 5-FU for neoadjuvant therapy of rectal cancer? DR LOVE: In a clinical setting, how do you decide between capecitabine
and infusional 5-FU for neoadjuvant therapy of rectal cancer? |
 DR TABERNERO: I give the option to the patient. I’m used to administering
5-fluorouracil as a continuous infusion, and I feel comfortable using it. But
a number of patients complain about having a pump continuously for six or seven weeks, so this is an issue. If patients have concerns about the pump, I
give them the opportunity to receive capecitabine as an alternative. DR TABERNERO: I give the option to the patient. I’m used to administering
5-fluorouracil as a continuous infusion, and I feel comfortable using it. But
a number of patients complain about having a pump continuously for six or seven weeks, so this is an issue. If patients have concerns about the pump, I
give them the opportunity to receive capecitabine as an alternative.
Select publications
|

