You are here: Home: CCU 2 | 2006: J Randolph Hecht, MD

Tracks 1-20 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Mechanism of action of small-molecule
tyrosine kinase
inhibitor PTK/ZK |
| Track 3 |
Dynamic contrast-enhanced
MRI as a surrogate marker for
response to PTK/ZK |
| Track 4 |
Mechanisms of VEGF inhibition |
| Track 5 |
Differences in the potential roles
of VEGF receptors 1, 2 and 3 |
| Track 6 |
Efficacy of single-agent
PTK/ZK |
| Track 7 |
Background and design of the
CONFIRM-1 and CONFIRM-2
trials |
| Track 8 |
Early primary endpoint analysis
of progression-free survival in
CONFIRM-1 |
| Track 9 |
Importance of pharmacokinetics
in the development of VEGF
inhibitors |
| Track 10 |
Correlation between progression-free
survival and LDH levels in
the CONFIRM trials |
|
| Track 11 |
Tolerability of PTK/ZK |
| Track 12 |
Potential mechanisms of action
of bevacizumab |
| Track 13 |
Potential rationale for bowel
perforations associated with
bevacizumab |
| Track 14 |
Incidence of arterial thrombotic
events with bevacizumab |
| Track 15 |
Continuation of bevacizumab
after disease progression |
| Track 16 |
Clinical use of bevacizumab
in the adjuvant setting |
| Track 17 |
Duration of treatment with
bevacizumab |
| Track 18 |
Clinical use of CAPOX in the
adjuvant setting |
| Track 19 |
TREE-1 and TREE-2 trials
evaluating oxaliplatin/ fluoropyrimidine
regimens as first-line
therapy for advanced colorectal
cancer |
| Track 20 |
Ongoing trials evaluating different
combinations of biologic agents
for advanced colorectal cancer |
|
|
Select Excerpts from the Interview
 Track 7 - 10 Track 7 - 10
 DR LOVE: Can you describe the CONFIRM trials, which studied the use
of PTK/ZK (vatalanib) in patients with untreated metastatic colon cancer? DR LOVE: Can you describe the CONFIRM trials, which studied the use
of PTK/ZK (vatalanib) in patients with untreated metastatic colon cancer? |
 DR HECHT: CONFIRM-1 enrolled previously untreated patients with
metastatic colorectal cancer (Hecht 2005), and CONFIRM-2 evaluated second-line treatment of patients with metastatic colorectal cancer. All patients
in the CONFIRM-1 study received 5-fluorouracil and oxaliplatin, administered
as FOLFOX-4. DR HECHT: CONFIRM-1 enrolled previously untreated patients with
metastatic colorectal cancer (Hecht 2005), and CONFIRM-2 evaluated second-line treatment of patients with metastatic colorectal cancer. All patients
in the CONFIRM-1 study received 5-fluorouracil and oxaliplatin, administered
as FOLFOX-4.
Patients were randomly assigned to receive either 1,250 mg of PTK/ZK daily
or placebo. The identical regimen was used in CONFIRM-2 for patients who
had failed treatment with 5-fluorouracil and irinotecan.
 DR LOVE: Can you discuss the trial results? DR LOVE: Can you discuss the trial results?
 DR HECHT: In both CONFIRM trials, progression-free survival was
measured rigorously using a central assessment, which was completely blinded
as to how the patient was doing clinically. DR HECHT: In both CONFIRM trials, progression-free survival was
measured rigorously using a central assessment, which was completely blinded
as to how the patient was doing clinically.
In CONFIRM-1, the final progression-free survival analysis showed a modest
improvement, which was not significant (HR = 0.88), but investigator-assessed
progression-free survival, a more common measurement, did reach
statistical significance (HR = 0.83; p = 0.026).
Progression-free survival was one of two primary endpoints; the other was
overall survival. At this time, we only have data for progression-free survival,
and that’s what was presented at ASCO 2005 (Hecht 2005).
One of the things seen in CONFIRM trials that I think was very interesting
was the correlation of progression-free survival and LDH, which has been
evaluated as both a prognostic indicator and a predictive marker for chemotherapy.
Historically, LDH has been used as a stratification criterion to make
certain both arms of a study are balanced. In these trials, LDH and performance
status were used as stratification criteria.
When you look at the patients who had high LDH – the worst-prognosis
group – they derived the greatest benefit from PTK/ZK. In fact, with the
addition of PTK/ZK, the hazard ratio of 0.88 decreased to 0.68 for centrally
assessed progression-free survival.
 Track 12 Track 12
 DR LOVE: I am curious about your thoughts on the antitumor mechanism
of action of bevacizumab. DR LOVE: I am curious about your thoughts on the antitumor mechanism
of action of bevacizumab. |
 DR HECHT: The original thinking was that bevacizumab inhibited the growth
of new blood vessels. In order for tumors to grow beyond a certain size, the
angiogenic switch is f lipped, and a mutation causes the secretion of growth
factors, which leads to the supply of new blood vessels to the tumor. DR HECHT: The original thinking was that bevacizumab inhibited the growth
of new blood vessels. In order for tumors to grow beyond a certain size, the
angiogenic switch is f lipped, and a mutation causes the secretion of growth
factors, which leads to the supply of new blood vessels to the tumor.
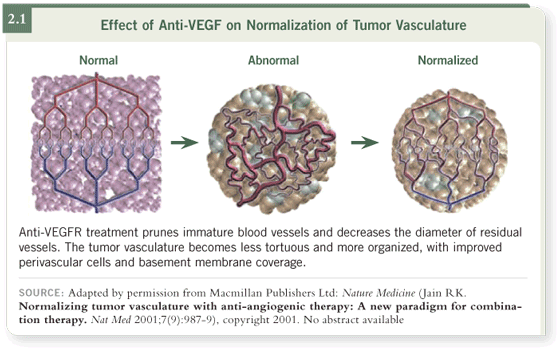
However, another name for vascular endothelial growth factor is vascular
permeability factor. Tumor blood vessels are leaky, and tumors tend to have
a high intratumoral pressure. One of the thoughts — this is sometimes called
the Jain hypothesis (Jain 2001) — is that the vasculature that’s associated with
tumors is abnormal. It’s tortuous. The vessels are dilated, inefficient, and also
very leaky. By giving anti-angiogenic therapy, you might be normalizing the
vasculature.
This process would have several effects (2.1), one of which could be to allow
a more efficient delivery of chemotherapy by providing more efficient blood
vessels and by reducing intratumoral pressure. It’s harder to get chemotherapy
in against a pressure gradient.
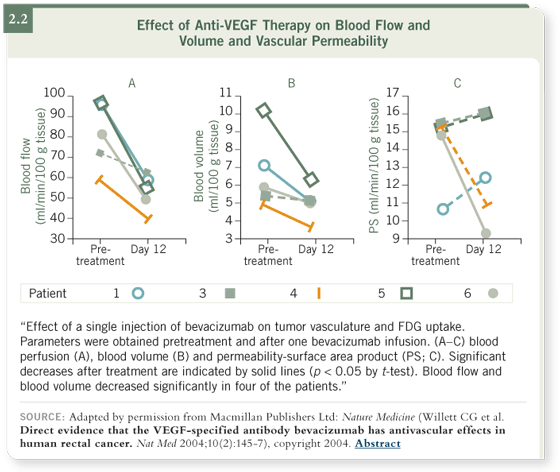
A study by Chris Willett (Willett 2004), published in Nature Medicine, had
a huge impact in terms of this thinking, although it had a small number of
patients. The study showed that following treatment of rectal cancer with
bevacizumab, blanching and shrinkage of the tumor occurred and less blood
(ie, perfusion and volume) appeared to be going to the tumor (2.2).
In addition, interstitial pressure of the tumor fell and significant decreases in
microvascular density occurred. This observation was made using bevacizumab
alone, which offers some validation that at least a portion of the Jain
hypothesis appears to be correct.
Bevacizumab increases response rates, and no one expected anti-angiogenic
therapies to increase response rates. All we ever expected was to cause stabilization
of disease by keeping new blood vessels from growing. Instead, virtually
all the trials with bevacizumab have shown an increase in response rate.
In fact, the colon cancer trials — the original 5-FU trial that Kabbinavar
published (Kabbinavar 2003), the IFL study by Herb Hurwitz (Hurwitz 2004),
Kabbinavar’s other 5-FU trial (Kabbinavar 2005), the TREE-2 trial (Hochster
2005, 2006) — all show approximately a 10 percent improvement in response
rate with the addition of bevacizumab.
 Track 15 Track 15
 DR LOVE: Do you continue using bevacizumab in a patient who has
disease progression? DR LOVE: Do you continue using bevacizumab in a patient who has
disease progression? |
 DR HECHT: Nobody knows the best approach in this situation, and there
are studies in development right now to answer that question. One of the
problems has been that the standard of care in colon cancer has changed so
rapidly that it’s been difficult to catch up and fill in all the gaps. DR HECHT: Nobody knows the best approach in this situation, and there
are studies in development right now to answer that question. One of the
problems has been that the standard of care in colon cancer has changed so
rapidly that it’s been difficult to catch up and fill in all the gaps.
The ECOG-E3200 trial, which Bruce Giantonio presented at ASCO this year
(Giantonio 2005), showed that the combination of FOLFOX and bevacizumab
was better than FOLFOX alone in patients who were bevacizumab-naïve and
who had failed an irinotecan-containing regimen. However, that group of
patients no longer exists because there are very few patients who don’t receive
bevacizumab front line.
The answer depends on how bevacizumab works. If bevacizumab works only
by blocking the growth of new blood vessels, maybe giving it after disease
progression doesn’t make any sense.
However, if bevacizumab works by facilitating the delivery of chemotherapy,
perhaps it does make some sense.
 DR LOVE: What do you do in your own practice? DR LOVE: What do you do in your own practice?
 DR HECHT: I have a discussion with the patient. Theoretically, it could make
sense to continue treatment; however, more toxicity might occur. We try
to enroll the patient in a clinical trial, since we are in an academic medical
center, but I do present that option to my patients. DR HECHT: I have a discussion with the patient. Theoretically, it could make
sense to continue treatment; however, more toxicity might occur. We try
to enroll the patient in a clinical trial, since we are in an academic medical
center, but I do present that option to my patients.
 Track 17 - 18 Track 17 - 18
 DR LOVE: The AVANT trial, like the NSABP-C-08 trial, is considering
what might be the most interesting question in adjuvant therapy right
now: the use of bevacizumab. However, it is also comparing capecitabine
to infusional 5-FU. What are your thoughts on this? DR LOVE: The AVANT trial, like the NSABP-C-08 trial, is considering
what might be the most interesting question in adjuvant therapy right
now: the use of bevacizumab. However, it is also comparing capecitabine
to infusional 5-FU. What are your thoughts on this? |
 DR HECHT: I believe that this will provide another therapy option. Personally,
I have patients who are very happy with infusional 5-FU, even when given
the choice between the two agents. DR HECHT: I believe that this will provide another therapy option. Personally,
I have patients who are very happy with infusional 5-FU, even when given
the choice between the two agents.
Other patients may prefer not to have a central line or may not want to wear a
pump, but remember that oxaliplatin requires a central line anyway.
The issue that will remain unanswered following those trials is the optimal
duration of bevacizumab. One thought was that if you were preventing the
growth of new blood vessels, maybe bevacizumab should be given to patients
for the rest of their lives.
Obviously, that’s not a practical option from either a toxicity standpoint or a
resource standpoint.
How long do you treat? Remember, we still don’t know how long to treat
with cytotoxic agents. Some data — British data, for example — suggest
shorter courses of chemotherapy may be as good as six-month regimens.
Not long ago, we were treating patients for a year. At this time, we’re
continuing the fluoropyrimidine/oxaliplatin and bevacizumab for six months.
 DR LOVE: Do you use capecitabine with oxaliplatin in the clinical adjuvant
setting? DR LOVE: Do you use capecitabine with oxaliplatin in the clinical adjuvant
setting?
 DR HECHT: I have done so in special circumstances. The data from an efficacy
standpoint showing functional equivalence between capecitabine-containing
regimens and infusional fluorouracil-containing regimens are good (Twelves
2005a, b). DR HECHT: I have done so in special circumstances. The data from an efficacy
standpoint showing functional equivalence between capecitabine-containing
regimens and infusional fluorouracil-containing regimens are good (Twelves
2005a, b).
Randomized Phase III trials of capecitabine alone versus 5-fluorouracil have
been conducted (Twelves 2005a, b), so the use of capecitabine in this setting is
not a huge extrapolation.
Select publications
|

