You are here: Home: CCU 3 | 2005: Alan
Venook, MD
| Alan Venook, MD |
EDITED COMMENTS |
 NSABP-C-09 trial: CAPOX with or
without intrahepatic FUDR NSABP-C-09 trial: CAPOX with or
without intrahepatic FUDR
The NSABP has been bold in their research
strategy, and in some cases that has had
a huge positive impact. Two major studies
suggest that intrahepatic FUDR following
resection is beneficial; however, those studies
were performed in an era before oxaliplatin
and irinotecan. As a proponent of regional
chemotherapy, I have no doubt that intrahepatic
FUDR will be a positive addition to these
combinations. Selecting the right patients for
the study could make a difference, but to their
credit, the NSABP is attempting to address
that issue (3.1).
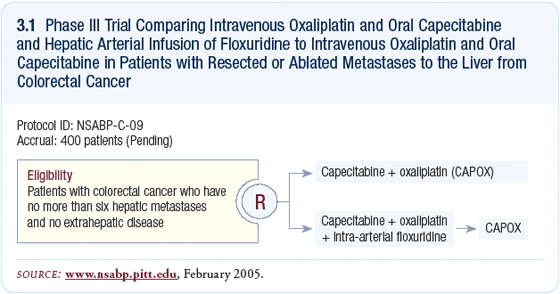
CAPOX (capecitabine/oxaliplatin)
At UCSF, we lean toward FOLFOX rather than CAPOX because we have data
for FOLFOX, and the current data are not adequate to say that CAPOX and
FOLFOX are equivalent. In practice we have seen robust responses with CAPOX,
FOLFOX, FOLFIRI and CAPIRI. Although we need more data, I do not anticipate that capecitabine will be a compromise for patients. The problem we have had
with CAPOX has been dosing, because it can cause hand-foot syndrome. We are
relatively conservative in our use of capecitabine and tend to favor it in elderly
patients.
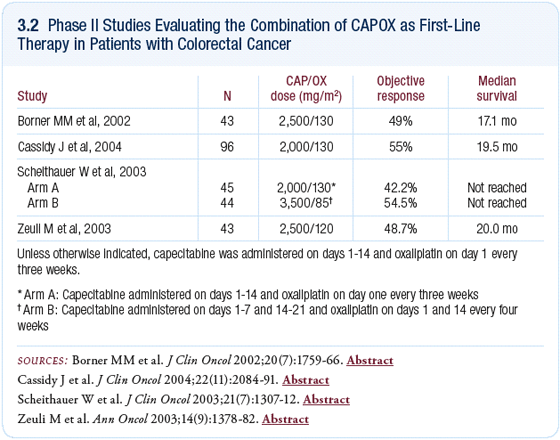
Whether research resources should be invested in investigating capecitabine in
combination with either irinotecan or oxaliplatin is a good question (3.2). On
one hand, with the new agents that need evaluation, it seems absurd to expend
resources on proving the equivalence of combinations of capecitabine versus 5-
FU. On the other hand, this has a huge impact on quality of life and patient satisfaction.
In an ideal world, we would enroll more patients with colorectal cancer
in clinical trials and be able to answer all of these questions.
Side effects of bevacizumab
Hypertension is a toxicity associated with bevacizumab, but it is almost always
easily managed with oral agents. Initial concern arose over whether bevacizumab
caused proteinuria, yet studies now indicate that only about 20 percent of
patients develop proteinuria. A potential safety issue related to bevacizumab is
the occurrence of bowel events. In patients who have had recent primary surgery,
the anastomoses may be compromised by the antiangiogenic action of bevacizumab
(Hurwitz 2004). That raises concern regarding the use of bevacizumab
in the adjuvant setting. In these trials, we will need to “watch like a hawk” to
ensure that bowel events do not become problematic.
Selection of patients with Stage II disease for adjuvant
chemotherapy
The NSABP philosophically believes that the benefit from chemotherapy is
accrued to patients with both Stage II and Stage III disease. The MOSAIC
trial demonstrated a statistically significant benefit in three-year disease-free
survival with FOLFOX versus infusional 5-FU/leucovorin in patients with
Stage III disease; however, statistical significance was not yet reached in Stage II
disease (de Gramont 2003).
In my opinion, the flaw in treating patients with Stage II disease in the NSABPC-
08 trial evaluating FOLFOX with and without bevacizumab is the accumulating
evidence that a subset of patients with Stage II disease should not be
subjected to the risk of chemotherapy.
ECOG is addressing that issue with a clever trial design that risk stratifies patients
with node-negative disease. This stratification is based on the molecular features
of the tumors. For example, patients who have normal 18q are observed without
therapy, based on retrospective data from a number of studies suggesting that
those patients do well, while patients in the study who have deletion of 18q are
randomly assigned to chemotherapy.
A relative risk reduction occurs with colorectal cancer chemotherapy, so the issue
lies in identifying the baseline risk. FOLFOX causes neuropathy, so in a patient
with node-negative disease who may have an 82 percent likelihood of being alive
and disease free five years later, you have to balance the benefit with the longterm
consequence.
Nonprotocol use of adjuvant chemotherapy
Based on the results of the MOSAIC trial, we have switched from using
5-FU/leucovorin to FOLFOX as adjuvant treatment for node-positive colon cancer
in the nonprotocol setting. Uncertainty lies in the important issue of how to treat
patients who develop neuropathy three months into therapy. The Intergroup
adjuvant study is going to sequence FOLFOX and FOLFIRI.
For patients with Stage II disease in the adjuvant setting off protocol, we present
all of the options, and the decision boils down to the patient’s philosophy.
Statistical estimates indicate that a study would require 4,000 patients to discern
a meaningful difference in chemotherapy effect in patients with node-negative
disease, but I don’t believe that such a study will be conducted.
Another important issue is the correlation of three-year disease-free survival
with five-year overall survival in clinical trials. I am comfortable that three-year
disease-free survival is a reasonable surrogate to predict benefit.
Select publications
 |
| Dr Venook is a Professor of Clinical Medicine, Associate
Chief of the Division of Medical Oncology and Director of the Clinical
Research Office at the UCSF Cancer Center in San Francisco, California. |
|

