You are here: Home: CCU 3 | 2005: Steven
A Curley, MD
| Steven A Curley,
MD |
EDITED
COMMENTS |
 Radiofrequency ablation for patients with
liver metastases Radiofrequency ablation for patients with
liver metastases
I began studying this modality in the laboratory in 1993
and in clinical trials in 1996. It’s a technique that
allows us to treat tumors that cannot be resected because
they were either in a bad location or bilobar.
We’ve now shown that you can safely do a combination
of resection of the dominant or large tumors and then ablation
of the smaller tumors in the opposite lobe without an increase
in the complication rate. Long-term outcomes data with that
type of aggressive approach indicate good results with patients
surviving for longer periods of time (Pawlik 2003).
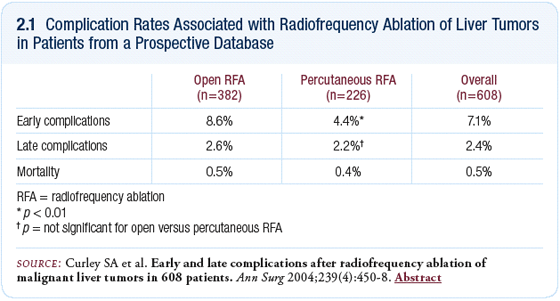
Radiofrequency ablation has a low rate of side effects;
our group has shown a less than 10 percent complication rate
(Curley 2004; [2.1]). In addition to the early complications
(eg, abscess in the ablated lesion or bleeding from the needle
track), some patients develop late complications such as
bile duct strictures or fistulae.
Others patients develop bilomas, which are large collections
of bile in the liver. Fortunately, those types of side effects
have a low incidence — only about 2.5 percent of patients
develop long-term side effects or toxicity (Curley 2004).
Radiofrequency ablation in clinical practice
This approach is now widely used in the United States.
It’s a technique that is being used by surgeons and
interventional radiologists. The incidence of local recurrence — what
I call incomplete treatment — is much lower in the
hands of surgeons, primarily because we do it intraoperatively
either with laparoscopic or open ultrasound guidance.
It is important to carefully evaluate the indications for
radiofrequency ablation. I use it only in patients who can
be treated with curative intent alone or combined with surgical
resection. I’ve seen patients who have undergone radiofrequency
ablation for palliation of symptoms. That may be useful in
select settings, but it has to be used judiciously.
In general, if the tumor can be surgically resected, that’s
what I do. In patients with colorectal cancer, with the techniques
we now have available, less than five percent of patients
require blood transfusions. Historically, liver resections
were associated with a high risk of problems. At MD Anderson,
the mortality rate is less than one percent and the complication
rate is less than 30 percent.
Maximizing the benefit of radiofrequency
ablation
We’ve seen the greatest benefit from ablation in
two patient populations. The first is the patient with disease
that is metastatic to the liver in a bad location (eg, nestled
on the vena cava or under the hepatic veins). Surgical data
demonstrate that unless you can perform a resection and obtain
a tumor-free margin, you do not provide any benefit to the
patient.
A tumor in that location frustrates surgeons because we
know we’re not going to be able to obtain a negative
margin. In that patient population, we can demonstrate a
benefit by performing tumor ablation with either radiofrequency
or microwave.
The other patients who will benefit from ablation are the
patient with hepatocellular cancer. The vast majority of
them have underlying liver disease, such as cirrhosis from
chronic hepatitis B or C infection, and they definitely have
some element of hepatic dysfunction.
Those patients are clearly at a high risk of liver failure
and death after a resection. Because we have such a high
demand for liver transplants in this country, many of them
aren’t going to be candidates for a transplant because
an organ will not be available.
We’ve just published our data from MD Anderson on
radiofrequency ablation for early-stage hepatocellular cancer,
and the results are actually better than the results after
resection but not quite as good as the results after transplant.
We’re now using radiofrequency ablation as a bridge
to transplantation in select patients.
Chemotherapy and radiofrequency ablation
in patients with liver-only metastases
In patients with colorectal cancer, using radiofrequency
ablation alone without any additional systemic or regional
chemotherapy offers about a 15 percent to 20 percent probability
of cure for unresectable disease. That’s based on our
own results at MD Anderson where our four-year overall survival
rate is about 22 percent. Certainly, a subset of patients
will be alive and without disease at four years (Abdalla
2004).
Adding chemotherapy to radiofrequency ablation — either
as neoadjuvant or adjuvant therapy — nearly doubles
the overall survival to 35 percent to 40 percent. In contrast,
if those patients were treated only with systemic chemotherapy,
that number would be less than 10 percent (Abdalla 2004).
In the past, it would have been less than one percent or
two percent, but the response rates are higher, with some
of the FOLFOX regimens and the addition of drugs like bevacizumab.
Some patients will refuse chemotherapy or will have had
previous chemotherapy and do not want more. That doesn’t
mean we won’t offer them a resection. We know that’s
going to give them their highest probability of long-term
survival, but we tell them they’re going to need to
be followed closely.
NSABP-R-04: Capecitabine versus continuous
infusion 5-FU as neoadjuvant therapy for rectal cancer
A large volume of Phase II data demonstrates that capecitabine
is similar to infusional 5-FU. While it’s a good idea
to perform the NSABP study, I’m not sure that we necessarily
need it. It’ll be interesting to see how that study
accrues. In this country, physicians are rapidly adapting
regimens based on Phase II data, but it’s become a
bit of a minefield because so many regimens are available.
I think R-04 is going to be sort of a “thanks for confirming
what we already suspected was true” type of study.
Select publications
 |
| Steven A Curley, MD Dr Curley is
a Professor of Surgical Oncology and Chief of Gastrointestinal
Tumor Surgery at The University of Texas MD Anderson
Cancer Center in Houston, Texas. |
|

