You are here: Home: CCU 3 | 2005: John
L Marshall, MD
| John L Marshall,
MD |
EDITED
COMMENTS |
 Efficacy
of bevacizumab in combination with chemotherapy in first-
and second-line clinical trials Efficacy
of bevacizumab in combination with chemotherapy in first-
and second-line clinical trials
The ECOG E3200 trial, which randomly assigned patients
with previously treated advanced colorectal cancer to receive
FOLFOX4 with or without bevacizumab, demonstrated a positive
survival advantage with the addition of bevacizumab (Giantonio
2005).
These data could have an immediate impact on the clinical
use of bevacizumab, particularly in patients with refractory
disease or in second-line therapy.
Previously, FOLFOX had not shown an independent survival
advantage in the second-line setting. In Rothenberg’s
study, FOLFOX showed improvement in time to progression,
but not survival (Rothenberg 2003). In E3200, patients who
received FOLFOX alone clearly did as well as patients who
received FOLFOX in Rothenberg’s study. In E3200, the
addition of bevacizumab increased survival by almost a couple
of months.
In the IFL plus bevacizumab front-line trial, a dramatic
improvement occurred in survival and time to progression
in patients who received the combination (Hurwitz 2004).
Trials such as these support our argument that adding bevacizumab
to chemotherapy regimens in the first- or second-line setting
can result in a positive outcome for patients in terms of
survival and progression-free survival.
The magnitude of benefit was greater in the front-line
study, and we don’t know whether that is because of
some front-line phenomenon that wouldn’t be seen in
second-line metastatic colon cancer or if something more “additive” occurs
with bevacizumab and irinotecan compared to oxaliplatin.
Synergy between bevacizumab and chemotherapy
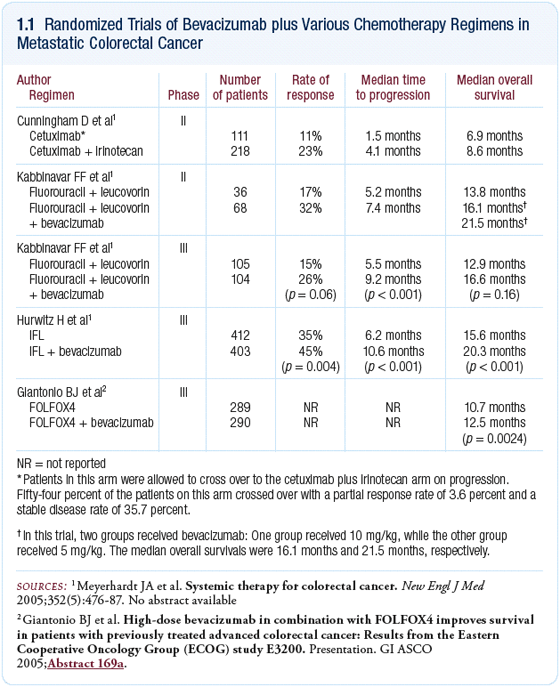
The front-line study examining 5-FU/leucovorin with or without
bevacizumab in patients with a poor performance status demonstrated
an advantage from the combination (Kabbinavar 2004). Clearly,
synergy exists between bevacizumab and 5-FU, and it appears
that when you start combining it with other chemotherapeutic
agents, that benefit carries over or adds up even further
(1.1).
What may be happening with bevacizumab in a number of settings
is that it controls the growth of the cancer, prevents progression
and, therefore, adds to progression-free survival, which
is now translating into overall survival.
I believe bevacizumab works by changing the dynamics of
the interstitial pressure within the tumor, which facilitates
the delivery of chemotherapy. If indeed that is its mechanism
of action, then the particular chemotherapy utilized shouldn’t
matter, and bevacizumab should improve its efficacy.
Continuation of bevacizumab after progression
One question the E3200 trial does not answer is whether
a patient who progresses on a front-line combination of chemotherapy
plus bevacizumab should be maintained on bevacizumab in the
second- and third-line settings.
The suspected mechanism of action of bevacizumab suggests
that patients should be maintained on this agent, but other
factors — including cost and side effects — must
be considered. If a patient is tolerating bevacizumab, I
believe it would be difficult to justify discontinuing it
when switching from a front-line to a second-line therapy.
Even though E3200 doesn’t answer that question directly,
I believe continuing bevacizumab is a reasonable strategy
in the nonprotocol setting, given the survival data in the
front- and second-line settings.
Nonprotocol use of first-line FOLFOX with
bevacizumab
I was surprised that after the data presented at the 2003
ASCO meeting on bevacizumab/IFL, clinicians shifted towards
using FOLFOX plus bevacizumab (Hurwitz 2004). I believe this
occurred because the FDA gave free reign to use bevacizumab
with any intravenous 5-FU-containing regimen. The pendulum
was already swinging toward using FOLFOX as the standard
front-line approach for patients with metastatic colon cancer,
so oncologists went ahead and combined it with bevacizumab.
That requires two leaps of faith — first, infusional
5-FU in FOLFOX rather than bolus 5-FU in the IFL regimen,
and, second, oxaliplatin rather than irinotecan — without
a lot of supportive data. The data from the E3200 trial support
both leaps, showing bevacizumab to be safe and effective
with infusion 5-FU and oxaliplatin.
Rationale for adjuvant clinical trials
combining FOLFOX and bevacizumab
To date, drugs that are effective in metastatic disease
are also effective in the adjuvant setting, so the obvious
next step after E3200 is to test FOLFOX plus bevacizumab
as adjuvant therapy. Based on the MOSAIC trial data, FOLFOX
offers clear benefit in the adjuvant setting (de Gramont
2005), whereas the IFL data were negative in the adjuvant
setting.
The NSABP is undertaking a study of FOLFOX plus bevacizumab
versus FOLFOX alone. As it stands now, patients will receive
12 months of bevacizumab — six months concurrent with
chemotherapy, followed by an additional six months of bevacizumab
alone — hoping for an antiangiogenic effect on microscopic
disease and the prevention of relapses.
The Intergroup is initiating a key trial for patients with
Stage III disease that will have three arms. One arm will
utilize FOLFOX as the standard of care, a second arm will
utilize 12 cycles of fluorouracil/leucovorin with or without
irinotecan (FOLFIRI), and the third arm will utilize six
cycles of FOLFOX and six cycles FOLFIRI. Adding a biologic
agent has also been discussed. It’s an interesting
study evaluating whether we can double up on chemotherapy
and further improve outcome in the adjuvant setting; however,
this trial is contingent on a positive result in the PETACC-3
study, which is evaluating adjuvant FOLFIRI.
Adjuvant therapy for patients with Stage
II disease
It is interesting that we tend to back away from adjuvant
therapy in patients who have a lower risk, when it may be
more appropriate to do exactly the opposite. Those are the
patients with whom we should be the most aggressive. In the
Stage II subset analysis of the MOSAIC study, the patients
who received FOLFOX had a three-year disease-free survival
of 87 percent. To my knowledge, that’s the highest
number ever reported for Stage II patients, and it’s
a compelling number in the clinic.
The real issue with using 12 cycles of FOLFOX in the adjuvant
setting is the high rate of neurotoxicity seen at the end
of that six-month treatment period. It’s not life threatening,
but it’s a nuisance for patients.
In breast cancer we are accustomed to utilizing adjuvant
chemotherapy for relatively small gains, meaning two to four
percent absolute gain. I believe we should be equally aggressive
when treating patients with colon cancer, and we should incorporate
these adjuvant therapies as often as possible. By adopting
these new therapies, we’re going to cure more patients
of this disease.
Safety and efficacy data from the X-ACT
adjuvant trial
The X-ACT trial was conducted in Europe with approximately
2,000 patients, half of whom received full-dose capecitabine — 1,250
mg/m2 bid, two weeks on, one week off — and
the other half received the Mayo Clinic 5-FU/leucovorin regimen
(Cassidy 2004b). Both of those recipes are considered “too
spicy” in the United States, so we were surprised when
the safety data revealed that approximately 60 percent of
patients did not require a dose reduction in either arm.
Although the remaining approximately 40 percent in both
arms needed dose reduction — either due to hand-foot
syndrome or the more toxic side effects of the Mayo Clinic
regimen, including neutropenia, sepsis and diarrhea. However,
the capecitabine arm was significantly less toxic.
The trial was designed to be an equivalence study, but
the analysis showed capecitabine to be superior by a couple
of percentage points in both disease-free and overall survival
(Cassidy 2004a). Therefore, based on the lower toxicity and
slightly higher efficacy data, I believe capecitabine is
a better option than the Mayo Clinic 5-FU/leucovorin regimen.
Capecitabine in the adjuvant setting for
Stage II colon cancer
Often when discussing treatment with young, healthy patients
with lower-risk Stage II disease, I consider using infusion
5-FU or sometimes even FOLFOX, but some patients don’t
want to approach it that aggressively, and some oncologists
even debate whether to offer chemotherapy to those patients.
I believe capecitabine, although controversial, is a good
option. I recognize that we don’t have firm randomized
trial data with capecitabine in Stage II disease, but in
the X-ACT study, capecitabine was equivalent to bolus 5-FU,
so I believe it’s an option for patients with low-
to moderate-risk Stage II disease.
Adjuvant chemotherapy for Stage III disease
In patients with Stage III disease, I generally use FOLFOX6
as adjuvant therapy because I believe the two-day infusion
schedule is the optimal way to administer 5-FU intravenously.
I discuss the MOSAIC and X-ACT data with the patients, and
some patients ask about using CAPOX. I usually steer them
toward one of the other options. I have treated patients
with CAPOX in the adjuvant setting, and my intuition tells
me that regimen is adequate so I would rather give that than
nothing; however, we need the trial data to be certain.
Select publications
 |
| Dr Marshall is an Associate Professor and
Director of Developmental Therapeutics and GI Oncology
at Lombardi Comprehensive Cancer Center at Georgetown
University in Washington, DC. |
|

