You are here: Home: CCU 1 | 2005: Norman Wolmark, MD
| Norman Wolmark, MD |
EDITED COMMENTS |
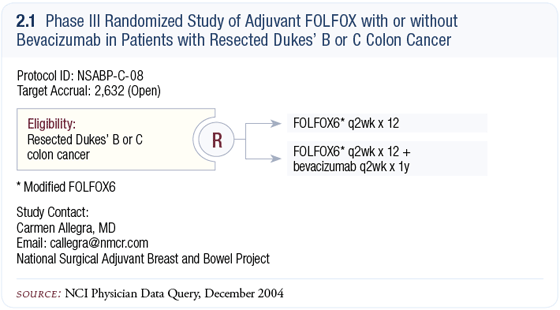
 NSABP-C-08: Phase III randomized trial of adjuvant FOLFOX with or without bevacizumab NSABP-C-08: Phase III randomized trial of adjuvant FOLFOX with or without bevacizumab
NSABP-C-08 was opened in October 2004, which from a regulatory standpoint is certainly a major accomplishment. The trial design is simple and straightforward — modified FOLFOX-6 with or without one year of bevacizumab. The eligibility criteria include patients with Dukes’ B or C colon cancer (2.1).
Originally, we wanted to make this trial as broad-based as possible and include FLOX (bolus 5-FU/leucovorin/oxaliplatin). The FDA didn’t particularly embrace that idea; their response was justified because we didn’t have data from NSABP-C-07. In view of the MOSAIC adjuvant trial data with a FOLFOX regimen (Andre 2004), I think a FOLFOX-inspired regimen is reasonable. So, we eliminated the possibility of having FLOX as a control arm. Also, we were thinking of including a capecitabine/oxaliplatin (CAPOX) arm, but the sample size would have been much greater.
Objectives of NSABP-C-08
We really wanted to address a pivotal question — whether the benefits associated with bevacizumab as first-line therapy for metastatic colorectal cancer can be translated to the adjuvant setting. Once we came to grips with that as our unequivocal principal aim, the trial was structured to address it. The sample size is manageable at about 2,600 patients. Theoretically, we hope bevacizumab will be more effective in the adjuvant setting. We hope the prolongation in time to progression seen in patients with advanced disease, if translated to the adjuvant setting, will result in lives saved.
Three-year, disease-free survival as a surrogate endpoint in adjuvant trials
We strongly support three-year, disease-free survival as a primary endpoint in adjuvant trials. Based upon the NSABP studies, we simply accepted that most of the events in patients with carcinoma of the colon occur within three years. I think we made an error in not disseminating that information widely and not recognizing that this information could have a significant impact.
Dan Sargent, in his combined analysis of 15 trials, unequivocally demonstrated that three-year, disease-free survival was an excellent surrogate for five-year survival (Sargent 2004). Unlike patients with breast cancer, most of the cancer events that occur in patients with colon cancer occur within the first three years.
We’re very comfortable utilizing three-year, disease-free survival as an endpoint for carcinoma of the colon, and I think more importantly, the FDA is comfortable with that endpoint. Virtually all of the trials that are currently ongoing and those that will be started, at least in the United States, are going to focus on disease-free survival.
NSABP-C-06: Phase III randomized adjuvant trial comparing oral UFT with leucovorin to 5-FU/leucovorin
We randomly assigned over 1,000 patients with Dukes’ B or C colon cancer to this trial. No differences were seen in disease-free and overall survival (Wolmark 2004). The results from NSABP-C-06, I think, are of interest.
UFT is not FDA approved, and it is not available in the United States. When Bristol-Myers Squibb went before the FDA to obtain an indication for UFT as first-line therapy in patients with metastatic colorectal cancer, two trials were reported — one by Douillard (Douillard 2002) and the other by Carmichael (Carmichael 2002).
Despite a unanimous ODAC (Oncologic Drugs Advisory Committee) recommendation to approve UFT, the FDA declined to act on that recommendation. As a result, UFT is not available. We were very disappointed when the FDA decided not to accept the ODAC recommendation.
NSABP-C-09: Phase III randomized trial of CAPOX with or without intra-arterial FUDR in patients with liver-only metastases
This new trial is for patients with liver-only metastases that are removed or ablated. Patients will receive CAPOX with or without intra-arterial FUDR. The European data with CAPOX for patients with liver-only disease certainly influenced the decision of the hepatic surgeons to use CAPOX as the baseline therapy. The question being tested is the role of intra-arterial FUDR. I think the real challenge is to see if hepatic surgeons from different institutions with different concepts can work together to evolve a clinical trial.
NSABP-R-04: Phase III randomized trial comparing preoperative therapy with capecitabine or continuous infusion 5-FU with or without oxaliplatin in patients with rectal cancer
The trial will be modified to include a randomization to plus or minus oxaliplatin, to determine oxaliplatin’s role in radiosensitization, achieving a pathologic complete response (pCR), preserving sphincters, and decreasing the localregional event rate at three years. This will be a two-by-two trial comparing capecitabine to continuous infusion 5-FU with or without oxaliplatin.
Select publications
 |
| Dr Wolmark is Chairman of the Department of Human Oncology at Allegheny General Hospital, Professor and Chairman at Drexel University College of Medicine and Chairman of the National Surgical Adjuvant Breast and Bowel Project in Pittsburgh, Pennsylvania. |
|

