You are here: Home: CCU 1 | 2005: Chris Twelves, MD
| Chris Twelves, MD |
EDITED COMMENTS |
 X-ACT adjuvant trial X-ACT adjuvant trial
Trial design and eligibility
The X-ACT trial (Cassidy 2004b) compared capecitabine versus bolus 5-FU/leucovorin as adjuvant therapy for colon cancer. Designed to mirror the metastatic trial, we hoped to prove that capecitabine was at least as effective in terms of disease-free survival, relapse-free survival and overall survival. The trial was multinational with 2,000 patients and it was limited to patients with Dukes’ C colon cancer. We excluded patients with rectal cancer because it’s biologically different, and the role of adjuvant therapy in rectal cancer is less clear.
We chose Dukes’ C because the rationale for and the benefits of adjuvant chemotherapy are clearer in patients with this disease stage. Specifically, the benefits of adjuvant 5-FU in Dukes’ C disease are quite significant and well demonstrated, so this criteria ensured a rigorous test for capecitabine. We were concerned that if we allowed patients with Dukes’ B disease, the study would be underpowered because a larger proportion of these patients are cured by surgery alone, and therefore, the benefits of 5-FU would be more subtle.
Efficacy data
The predetermined aim of the trial was to show equivalence in terms of disease-free survival, and that was achieved with a p-value of less than 0.0001. We designed the protocol so that if we achieved the primary endpoint, a secondary analysis of superiority could be triggered. This analysis was, indeed, performed on disease-free survival, and it just failed to reach statistical significance with a p-value of 0.05. However, the fact that we almost reached the secondary goal of showing superiority is a reflection of the degree by which we exceeded our stated endpoint.
Capecitabine reduced the risk of death by 16 percent and reduced the risk of recurrence by 14 percent. The relapse-free survival favored capecitabine with a statistically significant p-value of less than 0.05. The distinction between diseasefree and relapse-free survival is subtle — disease-free survival includes other cause deaths.
Not only did the disease-free, relapse-free and overall survival favor capecitabine, when we performed the multivariate analysis examining the prognostic factors that predict these outcomes, in each case the allocated treatment, capecitabine, was one of the factors that significantly influenced these endpoints. In terms of efficacy, adjuvant capecitabine proved to be at least as effective as, and may well be more effective than, bolus 5-FU/leucovorin.
Within our practice, a proportion of patients will be treated with combination chemotherapy as part of their adjuvant therapy, but it’s unlikely that will apply to all patients. Patients tolerate treatment differently and not all patients have the same risk of recurrence. I believe there will continue to be a role for single-agent fluoropyrimidines and, in that setting, there’s a strong argument now that capecitabine should replace the Mayo regimen. It’s at least as effective and more tolerable.
Safety data
The initial dose for capecitabine was 2,500 mg/m2, total dose per day, 14 days on, seven days off, and many patients required dose reductions. The proportion of patients requiring a dose reduction was very similar for the two arms of the study — 42 versus 44 percent for capecitabine versus 5-FU/leucovorin, respectively. When we evaluated the number of patients experiencing treatment interruptions, there were more with capecitabine, as we would expect.
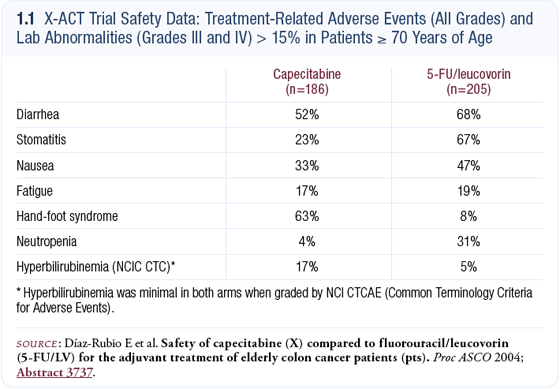
We view that as one of the advantages of capecitabine, in that this 14-day administration allows more opportunity to adjust the dose as needed. Overall, the pattern of toxicities favored capecitabine — there was less diarrhea, stomatitis, alopecia and myelosuppression (1.1). The only toxicity that increased with capecitabine was hand-foot syndrome, which we are now experienced at preventing and managing.
Pharmacoeconomic analysis
Interestingly, when we did a pharmacoeconomic analysis based upon practice in the UK, we were able to show a substantial financial savings. Although the cost of capecitabine is greater, it is more than offset by the reductions in administration costs and the costs of managing adverse events. We are in the fortunate position of finding that not only is capecitabine probably more effective, but it is also less expensive than the current standard treatment (McKendrick 2004).
Capecitabine plus oxaliplatin in the treatment of metastatic disease
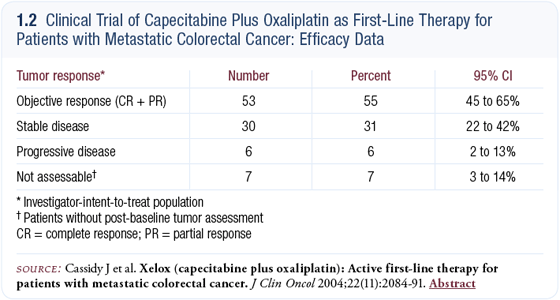
We have published Phase II data that show capecitabine/oxaliplatin (CAPOX) to be active as a first-line therapy for patients with metastatic colorectal cancer (Cassidy 2004a) (1.2). While this was not a randomized trial, there were nearly 100 patients and it was carried out in a number of centers. The data is quite robust compared to a small, single-institution study.
The overall objective response rate was approximately 55 percent and when we analyzed subgroups — such as age and sites of metastases — we saw very similar response rates in these groups. We also found that patients aged 65 and older were similarly as tolerant of the CAPOX regimen as younger patients, so we’re encouraged that this regimen can be used in a broader population and not just in the younger or fitter patients.
There have been a number of studies evaluating different schedules of CAPOX and different combinations with capecitabine in the metastatic setting, and they have broadly similar results. The studies of capecitabine/oxaliplatin and the capecitabine/irinotecan combinations demonstrate response rates of approximately 50 percent. Both combinations are well tolerated provided the appropriate dose modifications are made. Given the range of different combinations, settings and varying schedules for these studies, the data are astonishingly robust.
Off-protocol use of CAPOX in the adjuvant and metastatic settings
As one who participates in clinical trials, I prefer to wait for evidence from randomized studies before using new combinations off-protocol in the adjuvant and metastatic settings. However, with CAPOX I’m torn because everything we’ve seen to date from the clinical trials suggests that 5-FU can be substituted with capecitabine in these clinical settings. In addition, I would be very surprised if CAPOX doesn’t emerge as being equivalent to the FOLFOX regimen, alone or in combination with bevacizumab.
The broader question is, just how many times do we need to demonstrate the equivalence of 5-FU and capecitabine? We have used 5-FU in many different diseases and different combinations, and there has to be a limit, at least from the regulatory standpoint, to how many times equivalence has to be proven. I do believe CAPOX, off-protocol, is a reasonable option at this time.
MOSAIC adjuvant trial
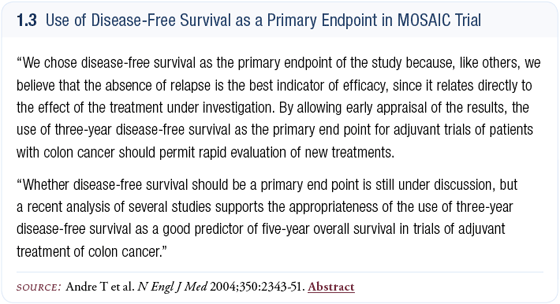
In the MOSAIC trial, the addition of oxaliplatin data resulted in a significant reduction in the risk of recurrence in the adjuvant setting (André 2004) (1.3). While the survival data is not yet mature, Sargent’s data, derived from a wide range of trials, clearly demonstrate that a delay in recurrence ultimately translates into prolonged survival (Sargent 2004).
Although the data are preliminary, I believe the MOSAIC data are the new gold standard. Only time will tell what that means for individual patients. A gold standard doesn’t necessarily mean the therapy applies to all patients. There are toxicities related to oxaliplatin, such as myelosuppression and neurotoxicity, and I don’t believe oxaliplatin-based adjuvant therapy will replace single-agent treatment across the board.
I anticipate a rapid move towards oxaliplatin-based treatments, especially in the younger, fitter and higher-risk patients. However, I believe a single-agent fluoropyrimidine will still be an appropriate option for a substantial proportion of older, more frail patients or patients at lower risk of disease recurrence.
Intravenous 5-FU therapy in the adjuvant setting
It’s difficult to identify a major role for intravenous 5-FU alone in the adjuvant setting as opposed to capecitabine. There will be some patients who are unable or unwilling to take tablets, or who can’t be relied upon to take oral medications. The point I often make is that if we had developed capecitabine first, no one would have developed intravenous 5-FU. Capecitabine has the convenience of an oral treatment that is at least as effective as 5-FU, and I believe it will increasingly become the backbone of treatment in colorectal and breast cancer.
Select publications
 |
| Dr Twelves is the NTRAC Professor of Clinical Cancer Pharmacology and Oncology at the University of Leeds and Bradford NHS Hospitals Trust in Bradford, United Kingdom. |
|

