You are here: Home: CCU 1 | 2005: Howard S Hochster, MD
| Howard S Hochster, MD |
EDITED COMMENTS |
 TREE trials: A comparison of three different oxaliplatin-containing regimens administered with or without bevacizumab TREE trials: A comparison of three different oxaliplatin-containing regimens administered with or without bevacizumab
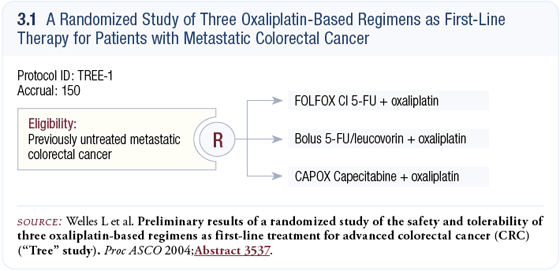
As far as we could tell from the Phase II and III studies conducted to date, there is significant activity of oxaliplatin with infusional 5-FU, bolus 5-FU or capecitabine. A huge study would be required to determine if one were better than the other; however, we believed significant toxicity differences could be observed in a smaller study. So, the TREE-1 trial looked at the three ways of administering oxaliplatin with a fluoropyrimidine (3.1) (Welles 2004).
After we accrued the first 150 patients to the TREE-1 trial, bevacizumab was shown to be effective in the pivotal trial with IFL. Therefore, we added bevacizumab to each of the three arms for the next 225 patients. Now, 375 patients have been randomly assigned to one of the three oxaliplatin-containing regimens administered with or without bevacizumab in a sequential design.
It’s not a true controlled randomized study of bevacizumab, but we will have comparative data for the different ways of administering the fluoropyrimidine with oxaliplatin — 150 patients without bevacizumab and 225 patients with bevacizumab. We’ll have some safety data to present at the ASCO GI meeting in January 2005 and a combined response analysis for ASCO in 2005.
The first thing we discovered in the TREE-1 trial was that the CAPOX regimen (oxaliplatin 130 mg/m2 every three weeks and two weeks of capecitabine 1,000 mg/m2 twice a day) was not well tolerated. We had a significantly higher incidence of diarrhea, dehydration, hospitalizations and dose reductions in that arm compared to the other arms. In October 2003, the Data Safety Monitoring Board (DSMB) recommended that the dose of capecitabine be reduced to 850 mg/m2 twice a day for two weeks.
For the TREE-2 study, the lower capecitabine dose was used in combination with bevacizumab. As far as we know from the DSMB analysis, the toxicity associated with the addition of bevacizumab is pretty much what people would expect, based on the already-known data from the bolus IFL/bevacizumab study (Hurwitz 2004b) and ECOG-3200 (Giantonio 2004).
Phase II randomized trial of bevacizumab and cetuximab with or without irinotecan in patients with irinotecan-refractory disease
We’re working with the New York Phase II consortium at Memorial Sloan- Kettering to complete a trial, with Leonard Saltz as the principal investigator, combining bevacizumab and cetuximab in patients whose disease has progressed on irinotecan. Patients are randomly assigned to receive both antibodies alone or both antibodies in combination with irinotecan.
This double-antibody study is a pilot trial for bringing both antibodies into the first-line setting. The patients on the study are tolerating the treatment very well. Even without irinotecan, the treatment seems to be holding the patients’ disease or causing shrinkages. I don’t know if or how much bevacizumab is adding to the cetuximab. The patients who receive the two antibodies alone have very minimal toxicity, except for skin rash.
First-line therapy in patients with metastatic disease
In a nonprotocol setting, I’ve been comfortable using an oxaliplatin-based regimen in combination with bevacizumab as first-line therapy for patients with metastatic disease, based on the TREE study and our own personal experience. The best data for improved time to progression, response rate and survival are with bevacizumab as first-line therapy, and I am most comfortable using oxaliplatin in the first-line setting. Therefore, I tend to use FOLFOX with bevacizumab in patients not enrolled on a protocol. We have seen nice responses and patients staying on those regimens for a long time.
Because of the issues with the dosing of capecitabine, concerns about compliance, and issues with diarrhea and hand-foot syndrome, I’m a little less likely to use capecitabine than a 5-FU infusion, unless the patient really objects to the infusion. We have patients we treat with CAPOX, and I’m conducting a study with a variation of the CAPOX regimen as first-line therapy. A role for capecitabine definitely exists, but we’re still learning how to use it most effectively in the United States.
Second- and third-line therapy for patients with metastatic disease who have received FOLFOX in combination with bevacizumab
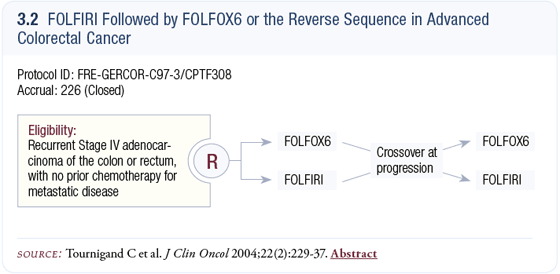
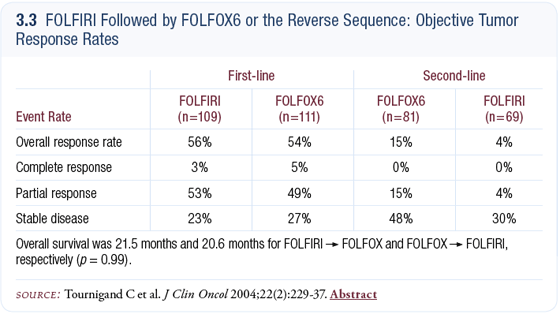
If I start patients on FOLFOX plus bevacizumab, then second-line therapy becomes a bit of a question. No compelling data tells you exactly what to do in that setting. You could adopt FOLFIRI after FOLFOX, based on the European study by Chris Tournigand (Tournigand 2004) showing that the sequence for FOLFOX and FOLFIRI doesn’t matter (3.2, 3.3).
You could argue for the use of irinotecan alone. We know that 5-FU is synergistic with oxaliplatin from the second-line trial (Rothenberg 2003), but nobody has ever shown that with irinotecan. You might use irinotecan with cetuximab, even though that’s not strictly within the FDA-approved indication. Additionally, you might use either regimen with bevacizumab, because if it’s inhibiting angiogenesis or helping the chemotherapy enter the tumor, it probably would work as well in the second-line setting as in the first-line setting.
In general, based on the Tournigand data, I would tend to use FOLFIRI and probably continue bevacizumab, because I don’t think there’s a lot of additional toxicity. Of course, the risk of perforation and thrombotic events exists, but in otherwise healthy patients, I don’t think that’s a major risk. I think most patients are willing to accept that risk, if you discuss it with them.
Then, most often, I’ll go on to a cetuximab regimen after using two lines of chemotherapy with bevacizumab. Based on the data from the two US studies (Saltz 2001, Saltz 2004) and the one randomized European study (Cunningham 2004), you’re better off using irinotecan with cetuximab than cetuximab alone. The combination of cetuximab and irinotecan doubles the response rate and more than doubles the time to progression.
Adjuvant therapy for patients with Sta
welleducated
ge II colon cancer
I’ve been a little disappointed with the failure of the colon cancer community to pick up on the results of the adjuvant therapy trials, especially combination chemotherapy for patients with Stage II disease. Those who treat breast cancer have known for a long time that adjuvant chemotherapy works just as well for patients with node-negative disease and that the relative benefit is about the same; it’s just that the absolute benefit becomes smaller as the prognosis is better. If I see an otherwise healthy patient with Stage II colon cancer, I tend to offer them adjuvant FOLFOX.
If we were to treat all of the patients with Stage II colon cancer in the United States every year, we’re talking about 3,000 lives saved. In breast cancer, patients and doctors are willing to accept more toxicity for a one percent difference; we aren’t there yet in the colon cancer community.
I was very disappointed by the publication of the ASCO guidelines in the Journal of Clinical Oncology (Benson 2004), in which they did not recommend adjuvant therapy for patients with Stage II colon cancer. In Europe, it’s accepted pretty widely and I think we should be moving in that direction.
X-ACT: Adjuvant capecitabine trial in patients with Dukes’ C colon cancer
The X-ACT trial was a comparison of adjuvant capecitabine to the Mayo Clinic regimen. We now know adjuvant capecitabine is equal to or perhaps slightly better than the Mayo Clinic regimen (Cassidy 2004). I think that’s a very important observation, and adjuvant capecitabine is a reasonable option for a well-educated patient who can be relied upon to take pills on a regular basis.
This requires a highly motivated patient who will call you or come in when they start to develop diarrhea, hand-foot syndrome or any of the toxicities. I don’t have a hesitation to use adjuvant capecitabine, based on the clinical data at this point in the adjuvant setting.
Clinical trials with cetuximab
I have experience with cetuximab going back to the original study, presented by Len Saltz, in patients with irinotecan-refractory disease who received irinotecan with cetuximab (Saltz 2001). The fact that adding cetuximab could make approximately 20 percent of the patients respond again and actually have measurable shrinkage was impressive. I believe cetuximab is going to be effective as first-line therapy. A trial from Europe is suggesting a high response rate in the first few patients treated with FOLFOX plus cetuximab as first-line therapy (Tabernero 2004). In a large randomized study — the EXPLORE trial — patients whose disease has progressed on irinotecan-containing regimens will be randomly assigned to FOLFOX with or without cetuximab. That study will enroll 1,100 patients.
Select publications
 |
| Dr Hochster is Professor of Medicine and Clinical Pharmacology at NYU Cancer Institute in New York, New York. |
|

