You are here: Home: CCU 2 | 2004: Heinz-Josef Lenz, MD, FACP

Edited comments by
Heinz-Josef Lenz, MD, FACP |
Genetic profiling to predict response to 5-FU/platinum combinations
Using genetic profiling, we have accumulated significant data allowing us to predict response to chemotherapy in gastrointestinal cancers. It’s easier to predict nonresponse than response because it often takes only one pathway to be over- or underexpressed to render a drug ineffective, whereas all the genes need to be in place for it to be effective.
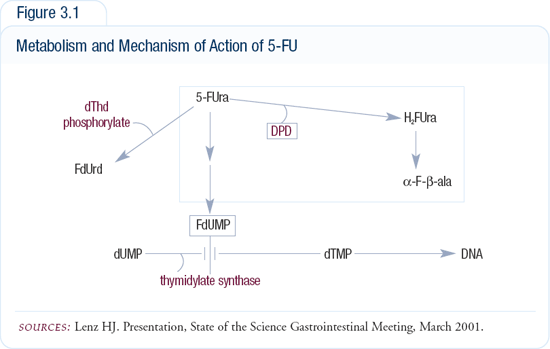
In the 5-FU pathway, we have identified three genes that can predict response (Figure 3.1). The target of 5-FU is the enzyme thymidylate synthase (TS), an essential enzyme for DNA synthesis. 5-FU is metabolized to FdUMP, which then binds in a suicidal manner to the TS protein. High levels of TS require high levels of 5-FU and some tumors have so much TS that we can’t administer enough of the drug because the toxicities are too high.
Another enzyme in the 5-FU pathway, dipyrimidine dehydrogenase (DPD), is clinically important because loss of this enzyme activity, which occurs in about one in one million cases, leads to life-threatening toxicities. These are the patients who, after one dose of 5-FU, die because they cannot detoxify 5-FU. On the other hand, if DPD is highly active, 5-FU gets detoxified and cannot inhibit TS. High levels of either TS or DPD are associated with resistance to 5-FU; both have to be low for response to this therapy.
We have similar genes to predict outcome in the oxaliplatin and cisplatin pathways. Platinums are cytotoxic by setting inter- and intrastrand DNA adducts, but a cell can defend itself by excisional repair, actually cutting off these adducts. Gastric and colorectal tumors with high levels of repair capacity, reflected in high levels of ERCC-1, are therefore resistant to cisplatin and oxaliplatin chemotherapy.
By looking at these three enzymes we can determine which tumors may be more responsive to 5-FU/platinum combinations. In a prospective study of the 5-FU pathway, we found that after excluding the patients with high TS and DPD levels, the response rate was over 80 percent. As for ERCC-1, when we have low ERCC-1 and TS, the likelihood of response is approximately 60 to 70 percent. The problem is in the patients who do not fall into these categories; we do not know what treatments to use. We only know that 5-FU or a platinum would likely be unsuccessful.
Genetic profiling to predict capecitabine efficacy and toxicity
Capecitabine functions in a manner similar to 5-FU, however, genetic activation is by thymidine phosphorylase (TP). When we added TP to the equation of TS and DPD, the predictive value went up to 100 percent. However, we expected high levels of TP would predict increased sensitivity to 5-FU and it’s just the opposite; high levels actually result in resistance to 5-FU treatment and there’s no biochemical explanation for it.
One hypothesis is that TP — also known as platelet-derived growth factor —plays a significant role in angiogenesis, and the role of angiogenesis overlays prediction of response to 5-FU. Many researchers believe the ratio of DPD and TP may be predictive for response to capecitabine, but we lack data from clinical trials to confirm this hypothesis.
Predictors of capecitabine-associated toxicities
The dose-limiting toxicities for capecitabine are mucositis and hand-foot syndrome. In our studies, the TS polymorphism predicted these toxicities. The cause of hand-foot syndrome is unknown, although there are theories that it may be caused by metabolites from the 5-FU, and particularly from the capecitabine pathway, and there are speculations that DPD plays an important role.
COX2 inhibition interferes with the prostaglandin pathway, so celecoxib has been combined with 5-FU to decrease the potential for diarrhea. What’s exciting is the potential effect on survival by COX2 inhibition. Data comparing capecitabine with or without celecoxib showed a survival benefit for the combination in patients with metastatic colon cancer.
Potential biological mechanisms for oxaliplatin-associated neurotoxicity
We have preliminary preclinical data identifying patients at high risk for neurotoxicity, a dose-limiting toxicity for oxaliplatin, which we are trying to verify in prospective trials. The etiology of neurotoxicity appears to be related to the repair capacity in the neuron and the channels of sodium and calcium that potentially regulate the interaction between oxaliplatin and the nerve.
Based on this, when treating patients with oxaliplatin, I offer them prophylactic magnesium and calcium. Approximately half of my patients accept it and the other half wait to see if they develop symptoms. I do not recommend any glutathiones to decrease neurotoxicity because glutathiones are a critical element in the pathway of platinum resistance.
SWOG-S0304: Neoadjuvant trial using molecular markers to select chemotherapeutic regimens for patients with rectal cancer
SWOG-S0304 was the first clinical trial ever to use TS, DPD and ERCC-1 to determine which chemotherapy regimen patients will receive (Figure 3.2). It is a neoadjuvant trial for patients with locally advanced, borderline resectable rectal cancer.
Initially they will receive chemotherapy, based on their genetic profile, followed by chemoradiation with capecitabine. If they respond they may then undergo surgical resection. We want to know if these three genes can actually increase the likelihood of response to therapy.
We felt the safety profile of capecitabine demonstrated in numerous Phase II trials made it a good choice for the chemoradiation portion of this study. However, I believe randomized trials need to be conducted before capecitabine can replace standard therapy. My colleagues in Europe have the opposite opinion — based on the same trials, they have already replaced infusional therapy with capecitabine.
If this trial is successful, the next step will be a prospective clinical study testing the value of TS, DPD and ERCC-1 in predicting response to 5-FU and oxaliplatin. Patients with higher likelihood of response to 5-FU and oxaliplatin will be randomly assigned to FOLFOX and other patients will be randomly assigned to oxaliplatin plus ironotecan versus FOLFIRI. This design would allow us to compare the nonselected versus selected patient population and evaluate how patients with an unfavorable genetic profile respond to treatment.

Select publications
|
| Dr Lenz is an Associate Professor of Medicine and Preventative Medicine, Director of the Colorectal Center and Director of the GI Oncology Program at the USC/Norris Comprehensive Cancer Center in Los Angeles, California. |
|

