You are here: Home: CCU 2 | 2004: Axel Grothey, MD

| Edited comments by Axel Grothey, MD |
Capecitabine versus 5-FU/leucovorin regimens
As first-line therapy, capecitabine and the Mayo Clinic regimen — bolus 5-FU/leucovorin —have comparable efficacy. However, they have different safety profiles. Capecitabine is associated with fewer potentially lethal complications, like neutropenic fever, and more hand-foot syndrome than bolus 5-FU regimens. Overall, the safety profile favors capecitabine over bolus 5-FU/leucovorin, which is an inferior 5-FU regimen. Whether capecitabine is comparable to infusional 5-FU regimens is not known.
Phase II trials of capecitabine/oxaliplatin (CAPOX)
The largest Phase II trial — an international trial conducted in Europe and Canada — enrolled 96 patients and evaluated a combination of capecitabine (1,000 mg/m 2 twice daily on days one through 14, followed by a one-week rest) and oxaliplatin (130 mg/m2 every three weeks). This CAPOX regimen produced a 55 percent investigator-rated response rate, which is comparable to the response rates observed with FOLFOX-4, -6 or -7 (Figure 2.1). The time to progression and overall survival, likewise, were very similar to those observed with infusional regimens.
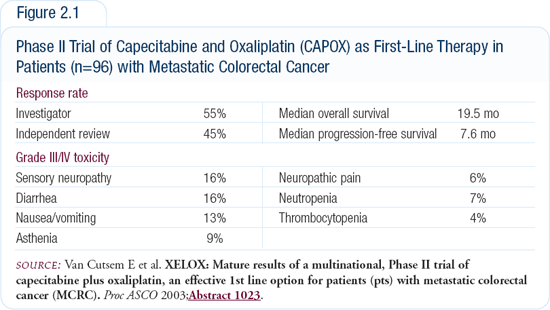
Another trial, conducted by our group in Germany, administered oxaliplatin on days one and eight. We had similar results with a greater than 50 percent response rate and a good toxicity and tolerability profile. The progression-free survival was more than seven months, and the overall survival is not yet available. Several other trials have also demonstrated that the CAPOX regimen is feasible and has about a 50 percent response rate and a more than seven-month progression-free survival.
Phase II randomized trial: Capecitabine/oxaliplatin (CAPOX) versus capecitabine/irinotecan (CAPIRI)
In a Phase II randomized trial comparing CAPOX to CAPIRI, we encountered early deaths in four out of the first 40 patients treated with CAPIRI. As a result, the irinotecan dose was reduced to 80 mg/m2 on days one and eight. Overall, five out of the 79 patients treated with CAPIRI died within the first 60 days. Two deaths were related to pulmonary embolism — a complication observed with IFL (irinotecan, bolus 5-FU and leucovorin), two deaths were related to septic diarrhea (i.e., diarrhea, neutropenic fever and sepsis) and one death may have been cardiac related. Altogether, the 60-day all-cause mortality rate was 6.5 percent for CAPIRI, which is in the range that has been reported with IFL. The 60-day all cause mortality rate for CAPOX was 1.2 percent.
Ongoing Phase III trials comparing capecitabine and infusional 5-FU regimens
It’s worthwhile to compare CAPOX and CAPIRI to their respective infusional regimens because capecitabine-containing regimens are so much more convenient for patients. Several trials on both sides of the Atlantic are currently comparing these regimens. Most of these trials have also added a biological agent. For example, SWOG-S0303 will compare CAPOX to FOLFOX-6 with or without bevacizumab in a two-by-two factorial design; EORTC-40015 will compare FOLFIRI to CAPIRI with or without celecoxib. Current challenges include the transition from infusional to oral 5-FU regimens and the incorporation of the new biologic agents (i.e., bevacizumab and cetuximab).
Selecting capecitabine versus infusional 5-FU regimens in a nonprotocol setting
In a nonprotocol setting, we have choices to offer patients and we can individualize treatment. Some patients do not want central venous lines, ports or pumps. Although Phase III trial data comparing capecitabine combination regimens and infusional 5-FU regimens are not available, if patients are informed about this lack of data and the results from the Phase II trials, then oral therapy can be discussed. I’ve used it and patients like it.
Phase III trial of IFL with or without bevacizumab
The bevacizumab trial results presented at ASCO 2003 were a great surprise. Bevacizumab was much more efficacious than I predicted; it will change our approach to patients with colorectal cancer. The problem with that trial was the use of IFL — a regimen no longer used in clinical practice because of the Intergroup trial results presented by Rich Goldberg.
Bevacizumab plus IFL generated an impressive overall survival advantage of about five months when compared to IFL plus placebo. In my opinion, time to tumor progression is the best parameter to assess the first-line efficacy of a treatment, because subsequent treatments may influence overall survival. In the bevacizumab trial, the time to tumor progression for patients treated with bevacizumab plus IFL was a “two-digit number,” the first ever such result reported in a Phase III trial. Looking at progression-free survival, the efficacy of bevacizumab in combination with chemotherapy was really impressive — a gain of four months. Additionally, the duration of response for the patients treated with bevacizumab plus IFL was 10.4 months, which was much longer than the duration of response for the patients treated with IFL plus placebo (Figure 2.2).
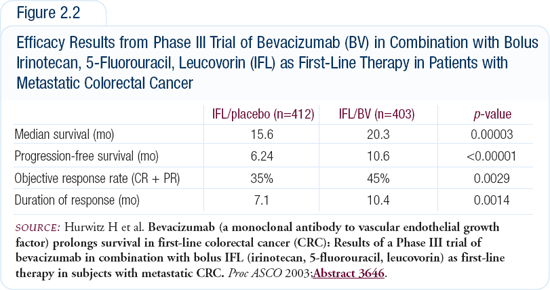
Initially designed as a three-arm trial, the third arm consisted of bolus 5-FU/leucovorin plus bevacizumab. When IFL plus bevacizumab was found to be a safe and feasible regimen, the third arm of the trial was closed to accrual so we have limited data about that regimen. Bolus 5-FU/leucovorin plus bevacizumab was better than IFL alone, in terms of response rate, progression-free survival and overall survival. The results for bolus 5-FU/leucovorin plus bevacizumab did not compare to the results for IFL plus bevacizumab, but bevacizumab added more than irinotecan to bolus 5-FU/leucovorin. This may also indicate that bevacizumab’s efficacy is independent of the chemotherapy regimen used.
Bevacizumab plus capecitabine
In patients with heavily pretreated breast cancer, a trial has compared bevacizumab plus capecitabine to capecitabine alone. Although the trial was negative, the group of patients treated with bevacizumab plus capecitabine had a 10 percent increase in response rate — the same as seen in patients with colorectal cancer. However, this did not translate into a gain in survival or progression-free survival. In patients with colorectal cancer, a SWOG trial will compare CAPOX with or without bevacizumab to FOLFOX with or without bevacizumab.
Use of bevacizumab as first-line therapy in patients with metastatic disease
My first impulse would be to use bevacizumab in combination with the best chemotherapy regimen — a FOLFOX regimen. However, very limited data exist for that combination. The second-line trial by ECOG, of FOLFOX-4 with or without bevacizumab, has completed accrual, but we don’t have data yet. It might be a negative trial because the second-line setting may differ from the first-line setting, irrespective of the efficacy of FOLFOX plus bevacizumab. I would like to see results from clinical trials with FOLFOX plus bevacizumab in the first-line setting. If they turn out to be positive, then that will be the best first-line therapy.
With the currently available data, bevacizumab plus FOLFIRI would be the most likely first-line therapy. It may be safe to use bevacizumab with FOLFIRI. FOLFIRI is better than IFL because it causes less toxicity and greater efficacy. I can’t see any reason bevacizumab plus FOLFIRI wouldn’t be as good, if not better, than bevacizumab plus IFL.
Select publications
|
| Dr Grothey is a Mayo Foundation Scholar at the Mayo Clinic College of Medicine in Rochester, Minnesota. |
|

