You are here: Home: CCU 2 | 2004: Richard M Goldberg, MD

| Edited comments by Richard M Goldberg, MD |
Adjuvant chemotherapy after resection of liver-only metastases
After a hepatic resection, I use chemotherapy as a standard in my practice, but I do it without sufficient data. A practicing oncologist must make many decisions without adequate data. I go by my instincts and try to draw conclusions from similar circumstances, either in the same disease or others.
In a small NCCTG trial (NCCTG-974651) of 44 patients with nonoptimally resectable liver-only metastases from colorectal cancer, 60 percent of the patients responded to FOLFOX (infusional 5-FU, leucovorin and oxaliplatin), and 17 were able to go on to surgery. Of those 17 patients, two had multiple small metastases throughout the liver that could not be resected. In one of the patients in whom the liver was resected, the tumor had a positive margin; the others had negative margins.
Unfortunately, in most of those patients the tumor recurred despite surgery. Of the patients who did not have a recurrence, all had received several cycles of chemotherapy after the resection. The median survival exceeded 30 months for the entire group, suggesting that surgery for patients whose tumors are resectable but not necessarily curable may still offer clinical benefit.
For patients with advanced disease, the N9741 trial established the superiority of FOLFOX over IFL (irinotecan, bolus 5-FU and leucovorin). In an analysis of that trial, 22 out of the 800 patients treated developed resectable disease. All of the patients who did not have tumor recurrence after resection had received some type of adjuvant chemotherapy.
Further investigation of the need for continuing chemotherapy after resection in these settings is necessary. The risk of recurrence, which exceeds 70 percent, depends on the number and distribution of lesions.
NSABP-C-09 adjuvant trial in patients with resected/ablated liver-only metastases
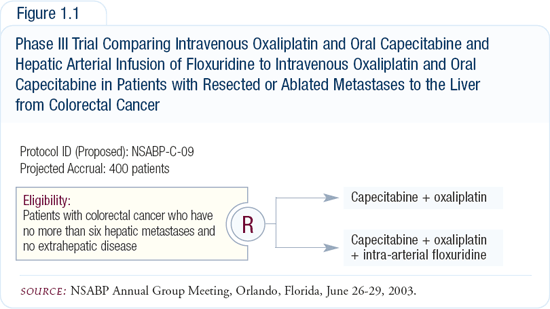
In patients with resected liver-only metastases, NCCTG conducted a pilot adjuvant trial (NCCTG-N9945) of oxaliplatin, capecitabine and a hepatic arterial infusion of FUDR (floxuridine). Based on that trial, NSABP-C-09 will randomly assign patients to oxaliplatin and capecitabine with or without a hepatic arterial infusion of FUDR (Figure 1.1). The trial will determine whether a hepatic arterial infusion is necessary with a better chemotherapy regimen.
Capecitabine has a long half-life and mimics a 5-FU infusion. Although preliminary Phase II trial data suggests that capecitabine may be equivalent to 5-FU infusions, Phase III trial data do not yet exist. In patients with advanced disease, it’s reasonable to substitute capecitabine for infusional 5-FU. In the adjuvant setting, I would prefer to see studies proving that 5-FU infusions and capecitabine are equivalent.
Theoretically, capecitabine may be advantageous because it is targeted 5-FU since higher levels of thymidine phosphorylase — the final activation step — are present in tumors compared to healthy tissue. Thymidine phophorylase levels may also be increased in the liver.
MOSAIC adjuvant trial
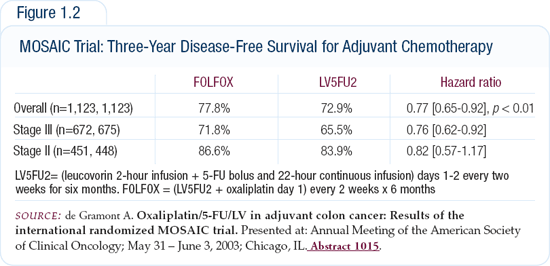
In patients with resected Stage II and Stage III disease, the MOSAIC adjuvant trial reported a 73 percent three-year disease-free survival for patients treated with 5-FU/leucovorin compared to a 78 percent three-year disease-free survival for patients treated with FOLFOX (Figure 1.2). Although patients with either Stage II or Stage III disease were included, patients with Stage III disease had a greater benefit.
Is three-year disease-free survival an adequate surrogate for five-year overall survival? My colleague Dan Sargent and I have submitted an abstract to the 2004 ASCO meeting showing that three-year disease-free survival is a very good surrogate and predictor of five-year overall survival. I believe the NCI has actually been willing to accept three-year disease-free survival as the primary endpoint for two new adjuvant trials on colon cancer in the United States — the NSABP and the Intergroup trials.
Future Intergroup and NSABP adjuvant trials
An Intergroup adjuvant trial (N0147) led by NCCTG will compare six months of FOLFOX, six months of FOLFIRI, and three months of FOLFOX followed by three months of FOLFIRI (infusional 5-FU, leucovorin and irinotecan). We also intend to randomly assign patients to cetuximab or no other therapy.
The NSABP adjuvant trial will compare FOLFOX with or without bevacizumab. The Intergroup colorectal cancer task force, which I chair, felt it was important to evaluate both cetuximab and bevacizumab in parallel in the adjuvant setting.
Rationale for incorporating cetuximab into adjuvant colorectal cancer trials
Cetuximab targets the epidermal growth factor receptor (EGFR) — a cell surface receptor that is present in tumors of all sizes. Even if just a few cancer cells were present, such as in the adjuvant setting, at least 60 percent would have measurable EGFR on their surfaces. The clinical trial data with cetuximab is based on two studies — one conducted in the United States by Leonard Saltz and the other conducted in Europe by David Cunningham.
In all of the trials the patients had been previously treated with irinotecan, and most were refractory to it. Patients were treated with either cetuximab alone or in combination with irinotecan. The response rates were about 10 percent for cetuximab alone and 23 percent for cetuximab and irinotecan.
These data are consistent and indicate that cetuximab has activity that is augmented when combined with irinotecan. Additional research is needed since we don’t know the response rates for cetuximab either alone or in combination with irinotecan and 5-FU as first-line therapy.
FOLFOX versus FOLFIRI in patients with metastatic colorectal cancer
When choosing between FOLFOX and FOLFIRI, I base my decision on my experience and the literature. Based on the Tournigand trial and other studies presented at ASCO 2003, the activity for FOLFOX and FOLFIRI appear to be very similar. However, FOLFOX and FOLFIRI have some subtle toxicity differences. FOLFOX is associated with more neuropathy and neutropenia. FOLFIRI causes more nausea, diarrhea and alopecia. To some extent, the patients’ comorbidities may factor into the decision.
Additionally, in patients with liver-only or lung-only disease, oxaliplatin-based regimens may be more effective in rendering them surgical candidates. We made that observation in an analysis of the CALGB-N9741 trial we submitted for the 2004 ASCO meeting. In my experience, the responses to oxaliplatin are sometimes more dramatic and rapid than the responses to irinotecan-based regimens. Patients who receive FOLFIRI or another irinotecan-based regimen may also become surgical candidates, but it seems more common with FOLFOX.
Incorporating capecitabine into the treatment of patients with colorectal cancer
While preliminary data suggest similar activity for capecitabine and infusional 5-FU, definitive data does not yet exist. I believe we will find that they are similar with perhaps different toxicity profiles. If capecitabine is utilized in a way to minimize toxicity, we’ll probably see similar efficacy. How the changes in Medicare reimbursement factor into the delivery of 5-FU and capecitabine will be interesting to observe.
In the neoadjuvant setting for patients with rectal cancer, it may be useful to compare capecitabine plus radiation therapy to infusional 5-FU plus radiation therapy. Soft data exist to suggest that radiation therapy may induce thymidine phosphorylase and, hence, improve capecitabine’s efficacy. Whether this is clinically meaningful will only be determined by clinical trials.
Select publications
|
| Dr Goldberg is a Professor, Chief of the Division of Hematology/Oncology and Associate Director of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina. |
|

