You are here: Home: CCU 1 | 2004: Mark S Roh, MD

Edited comments by Dr Roh, MD
NSABP-R-04 rectal cancer trial: Pre-operative capecitabine and radiotherapy
Rationale and design
While continuous infusion 5-FU in conjunction with radiotherapy is superior to bolus 5-FU with radiotherapy, the need for an intravenous catheter can cause associated problems and be a "hassle." NSABP-R-04 is a preoperative rectal cancer trial designed to evaluate capecitabine versus continuous infusion 5-FU. Patients in one arm are treated with capecitabine - mimicking a continuous infusion -while those in the control arm are treated with a typical course of the infusional 5-FU. All patients receive the same radiotherapy preoperatively and undergo surgery after systemic therapy.
We chose to evaluate capecitabine in this trial primarily because of its convenience. In addition, capecitabine preferentially concentrates in tumors up to three times as much as in normal tissue. In the two larger randomized studies of previously untreated patients with metastatic colorectal cancer, capecitabine was superior to continuous 5-FU infusion.
We will be collecting and storing tissue as part of this study. I believe years down the road, when we understand the molecular biology of rectal cancer, this information may help us find a prognostic indicator at a molecular level.
Novel strategies for trial accrual
Although the NSABP is very robust and vigorous, we realized that as an organization we cannot complete R-04 alone. We just don't have that many surgeons. We needed to reach out and involve those individuals not typically involved in these trials. In designing this study, we gathered a group of colorectal surgeons to assess the questions that were important to them and to enlist their help with accrual. In addition, we approached the major cooperative groups -ECOG, SWOG, CALGB, NCCTG - to work together on patient accrual and to hopefully advance the care of patients with rectal cancer.
This collaboration led to an agreement that NSABP-R-04 would be the first segment of a multisegment approach to patients with rectal cancer. Each of the cooperative groups would contribute to the preoperative R-04 trial, and this trial would be an entry point to postoperative trials from participating groups.
The protocol chairman, Bob Beart, is very well-respected and prominent in the colorectal community, and he has done a great job engendering the enthusiasm of his colleagues. In addition, to my knowledge there has not been a rectal trial that addressed the issues of colorectal surgeons or tried to elicit their participation. Hopefully, this will be a good experience in that regard.
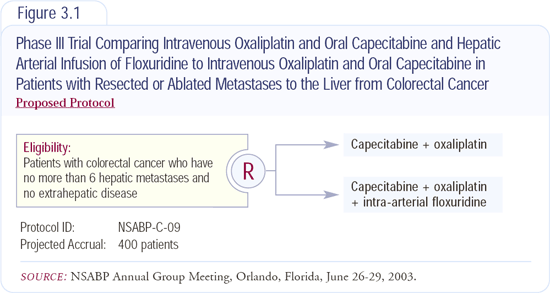
NSABP-C-09: Intra-arterial plus systemic therapy for resected hepatic metastases
In 1999, Nancy Kemeny published the results of an institutional trial showing that patients with hepatic metastases who undergo liver resection and receive both intra-arterial and systemic chemotherapy have improved survival and a decreased rate of recurrent disease within the liver. This was very exciting, and while theoretically it makes sense, it was only a single institution trial. Based on this trial, we have designed NSABP-C-09, which is a multi-institutional Phase III trial evaluating chemotherapy after liver resection for colorectal liver metastases (Figure 3.1).
Patients who undergo resection or ablation (or both) of six or less hepatic metastases will be randomly assigned to a combination of intra-arterial and systemic therapy or systemic therapy alone. Both groups will receive oxaliplatin and capecitabine as the systemic component of their treatment. Half of the patients will also receive floxuridine intra-arterially.
Floxuridine has been around for many years, has a high hepatic extraction, and we've not found a better drug. In terms of the choice of capecitabine and oxali-platin, we believe that capecitabine probably has efficacy at least equivalent to that of continuous infusion 5-FU. In addition, convenience was an issue. We believed that if the continuous infusion was too onerous for physicians, patients would not be enrolled in the study. With regard to the oxaliplatin, we tried to choose the best agent we could at the time, and we chose oxaliplatin over irinotecan.
The NCCTG are conducting a very similar Phase II trial in which everyone has the catheter placed and receives the same regimen of systemic drugs -floxuridine intrahepatically and then capecitabine/oxaliplatin afterward (Figure 3.2) . Essentially, this is a Phase II study of what will be one arm of our study. They are trying to accrue 50 patients, and the understanding is that if we help them with their trial, they will help with ours.

Surgical options for rectal cancer
Sphincter preservation and distance from the anal verge are key issues in determining the surgical procedure. The difficult cases are when the tumors are within three centimeters of the anal verge. The treatment of these patients is somewhat reflective of the experience of the surgeon. Some surgeons believe that is too close and that an abdominoperineal (AP) resection - a complete excision of the rectum, the distal rectum and the anus, requiring a permanent colostomy - is necessary.
A number of surgeons - and it's becoming more common - will excise almost down to the sphincter, and do a coloanal anastomosis to a pouch. The pouch maintains a reservoir so that patients are not going to the bathroom all the time. This procedure allows you to maintain oncologic principles, yet allows patients to resume somewhat more normal lifestyles than those who undergo AP resection.
In the best of hands, I believe the number of patients who undergo an AP resection is decreasing - probably down to approximately 10 percent. The surgeons I work with associate an AP resection as a sign of failure. But it is amazing and unfortunate that this is still the dominant procedure performed - probably 25 to 30 percent of patients with rectal cancer are having this type of procedure done. I believe that it will become less and less common as time goes on. My hope and expectation is that surgeons will become more adept at the pouch technique.
Management of hepatic metastases with surgical resection
In the evaluation and management of hepatic metastases, determination of resectability is critical. After confirmation that the disease is potentially resectable, extrahepatic disease must be ruled out by a thorough systemic evaluation. Assuming the patient has resectable disease on CAT scan and no evidence of extra-hepatic disease, surgical resection is the ideal next step, offering the best chance for cure. Resectable lesions should be treated surgically, and the unresectable lesions -deeper and riskier lesions - can be treated with nonresective methods like ablation.
There is a great deal of variability in how surgeons approach resection. One critical issue is margins. I'm constantly amazed at surgeons who basically shell out a tumor in the liver leaving a positive margin. Extensive data shows that a patient's survival with this approach is the same as if they had not undergone surgery. The other issue is the treatment of bilobar disease, which is very common. Some surgeons remove only the disease in one lobe, knowing that there is residual disease and anticipating further resection of the residual disease after hepatic regeneration has occurred. This approach makes no sense to me, as the liver regeneration occurring after the initial resection is almost like fertilizer for the remaining tumors.
When I began resecting liver metastases, most medical oncologists believed that only patients with one lesion should be considered for resection. This was based on well-established literature demonstrating that these patients have the best chance for cure. But as our techniques and results have improved, we can resect or ablate many more lesions - up to five or six. I believe more medical oncologists now consider whether resection or ablation is possible, even in patients with several lesions. These cases should at least be presented to a liver surgeon.
Select publications
|
| Dr Roh is Professor of Surgery at Drexel University College of Medicine and Chairman of Surgery at the Allegheny General Hospital in Pittsburgh, Pennsylvania. |
|

