You are here: Home: CCU 1 | 2004: David Cunningham, MD, FRCP

| David Cunningham, MD, FRCP |
Edited comments by Dr Cunningham
First-line therapy for metastatic disease
We've seen a major transformation in how we approach patients with metastatic disease over the past five to 10 years. Outcome has significantly improved, with average survival increasing from 6 to 7 months for patients who, in the past, didn't receive treatment for metastatic colorectal cancer, up to as much as 20 to 24 months. Most of that improvement can be attributed to the introduction of irinotecan and oxaliplatin into routine management of patients with metastatic disease.
Currently, in a nonprotocol setting, we tend to use combination chemotherapy as part of the first-line strategy. We usually combine capecitabine with oxaliplatin or irinotecan.
Capecitabine versus infusional 5-FU
The absolute purist would say you should use infusional 5-FU, either with oxaliplatin in the FOLFOX regimen or with irinotecan in the FOLFIRI regimen. At the Royal Marsden, we've been using infusional chemotherapy for more than 15 years, but we've moved away from it mainly because of patient preference and ease of administration of oral agents. The Phase II studies indicate a similar pattern of toxicity with capecitabine plus oxaliplatin or irinotecan compared to the infusional schedules, but without the need for placement of central lines or portacaths.
The majority of patients prefer an oral medication. Randomized trials comparing oral capecitabine or UFT with bolus 5-FU with a crossover between Cycle 1 and Cycle 2 have clearly shown that patients have a strong preference for the oral preparation.
Comparison of FOLFOX and FOLFIRI in the metastatic setting
It's difficult to choose between an irinotecan-based regimen and an oxaliplatin-based regimen, particularly in conjunction with either capecitabine or infusional 5-FU. The Phase III study conducted by Tournigand and his colleagues from France, comparing FOLFOX to FOLFIRI, didn't show much difference between the two regimens. Either regimen is acceptable, and the choice should be based on patient preferences. The North American Intergroup study comparing FOLFOX to IFL has resulted in a rapid change in practice.
Bevacizumab in the treatment of metastatic colorectal cancer
The data supporting the use of bevacizumab was generated by combining it with IFL. We'd all like the flexibility to use bevacizumab with a variety of combinations. The first one that comes to mind is FOLFIRI, which is clearly better tolerated than IFL and is probably more active. Second, we'd like the option of using bevacizumab with capecitabine and irinotecan (CAPIRI).
Of course, at the moment, we don't have any persuasive data suggesting that we should combine bevacizumab with the oxaliplatin-based regimen, although I think most people suspect that the biological effect observed in the bevacizumab randomized trial is less dependent upon irinotecan than just combining it with chemotherapy. The data with bevacizumab will radically alter how we approach the disease, and I think we all want to integrate it into the first-line strategy as soon as possible.
Clinical trial of cetuximab with or without irinotecan
At ASCO 2003, we presented the results of a randomized Phase II study of patients with metastatic disease whose tumors were resistant to irinotecan. Patients were randomly assigned to continue irinotecan and add in cetuximab, or to cetuximab alone. The rationale was to determine whether there was genuine synergistic activity between irinotecan and cetuximab or whether the response to cetuximab seen in prior studies was purely related to the cetuximab.
We found that approximately 20 percent of patients responded to the combination and approximately 10 percent responded to cetuximab alone. We also found significantly improved, progression-free survival in patients receiving the combination (approximately four months versus one-and-a-half months), although there was no impact on overall survival. This is partially due to a crossover effect; approximately 50 percent of patients randomly assigned to cetuximab alone went on to have irinotecan added if they either failed to respond or responded temporarily.
The MOSAIC and NSABP-C-07 adjuvant trials
We may be at another crossroads in colon cancer. At ASCO last year, Dr DeGramont presented the results of the MOSAIC adjuvant study, which randomized patients to 5-FU/LV versus 5-FU/LV and oxaliplatin. At three years, there was a significant improvement in disease-free survival in patients who received 5-FU/LV and oxaliplatin.
These data were received with great enthusiasm by the oncology community as the first good example in which two drugs were better than one in the adjuvant treatment of colorectal cancer. There are some reservations about the trial, in that Dr De Gramont reported that the five percent difference in disease-free survival was likely to translate into an improved survival in the future. That's an area for debate. Some clinicians say, "That's good enough for me. I'm going to use FOLFOX in all my patients." Others say, "The data are interesting, but I prefer to us FOLFOX only in patients at higher risk."
The NSABP will report on trial C-07, comparing 5-FU with or without oxaliplatin as adjuvant therapy. Hopefully, C-07 will further reinforce the MOSAIC trial results. For patients at higher risk, with multiple positive nodes, I discuss the MOSAIC trial results. Some patients opt to receive the combination while others stick with 5-FU/LV in the absence of survival data.
Use of capecitabine in the adjuvant setting
We are awaiting the results of two large, randomized studies to determine whether oral agents can be substituted for 5-FU/LV in the adjuvant setting. NSABP-C-06 compares uracil/ftorafur (UFT) plus leucovorin to 5-FU/LV. The X-ACT trial is comparing the Mayo Clinic regimen of 5-FU/LV with capecitabine.
The data regarding administration and tolerability of capecitabine were presented by Christopher Twelves at ASCO last year and very much confirmed what we've seen in the advanced-disease setting. Capecitabine was well-tolerated, and I think most physicians believe it will be equivalent to 5-FU/LV.
If you're scientifically evaluating the evidence, you'd have to conclude that we need to wait for the data before using capecitabine in the adjuvant setting. I've been very open and clear with patients. I've told them, "We don't have definitive results, but most people think capecitabine and 5-FU/LV will be equivalent." On that basis, I have a number of patients who have elected to receive adjuvant capecitabine.
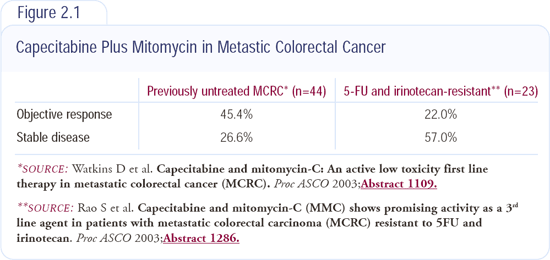
Capecitabine plus mitomycin for advanced colorectal cancer
Capecitabine is a prodrug of 5-FU, which is activated by three steps - the final one being thymidine phosphorylase (TP). It appears that TP is found at higher levels in tumor cells than in normal tissue, and this may enhance the conversion of capecitabine to 5-FU within the tumor cell. Mitomycin increases levels of TP within tumor cells, which might further enhance the effects of capecitabine.
At ASCO 2003, we reported data from two Phase II studies of capecitabine plus mitomycin (Figure 2.1). In the first study, patients who were unsuitable for standard combination chemotherapy received capecitabine and mitomycin as first-line treatment, and we had response rates of around 40 percent. In the second study, we evaluated the combination in patients who failed both 5-FU and irinotecan, and the response rate was approximately 17 percent. There are proposals to evaluate this combination in the UK in patients who failed both oxaliplatin- and irinotecan-based regimens.
As a nonprotocol treatment option for patients who do not have access to bevacizumab or cetuximab, it's certainly worth considering. Patients who have failed standard first- and second-line treatments are often desperate to receive any form of therapy.
Select publications
|
| Dr Cunningham is the Head of the Gastrointestinal Unit and Consultant Medical Oncologist at Royal Marsden Hospital in London, England. |
|

