You are here: Home: CCU 1 | 2004: Lee M Ellis, MD

Edited comments by Dr Ellis
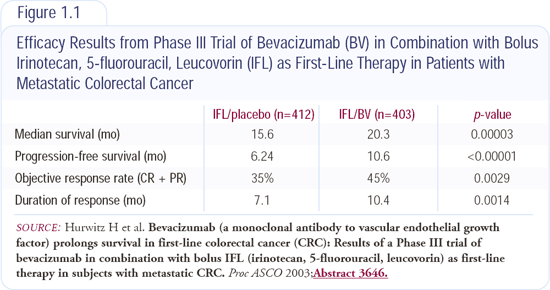
Phase III trial of IFL with or without bevacizumab
Dr Hurwitz's presentation at ASCO 2003 underscores the importance of investigating new agents and combining them with standard chemotherapeutic approaches. The addition of bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF), to standard chemotherapy - irinotecan, 5-FU and leucovorin (IFL) - led to a significant improvement in median survival from 15.6 months to 20.3 months. This was the first Phase III randomized study to demonstrate a benefit with antiangiogenic therapy (Figure 1.1).
Mechanism of action of bevacizumab
Thirty years ago, Judah Folkman first proposed the hypothesis that blocking blood vessel growth might inhibit tumor growth, since all tumor cells require a nutrient blood supply. Antiangiogenic therapy (e.g., anti-VEGF agents) inhibits further blood vessel growth and tumor growth; it does not necessarily decrease tumor size, as expected with standard cytotoxic chemotherapy. Therefore, the 10 percent improvement in response rate with bevacizumab seen in Dr Hurwitz's study and in the study with capecitabine and bevacizumab in patients with breast cancer was quite surprising. Interestingly, George Sledge reported a nine percent response rate in a Phase I/II trial of single-agent bevacizumab in patients previously treated for breast cancer.
A more complete understanding of the biologic mechanisms that led to this improvement in response rate is needed. Blocking new blood vessel formation shouldn't necessarily improve the response rate, but it may improve time to progression or overall survival. Other mechanisms of action for the anti-VEGF agents should be sought.
Vascular endothelial growth factor and tumor blood flow
Vascular endothelial growth factor (VEGF) was discovered as a vascular permeability factor, and it's the most potent permeability factor we have discovered. Tumors express high levels of VEGF, which leads to leakiness of the microvasculature within tumors and leakage of plasma proteins and fluid into the interstitial spaces. Since most tumors don't have lymphatics, this fluid cannot reenter the circulatory system. Therefore, interstitial pressure progressively increases as a tumor continues to grow - the larger the tumor, the greater the increase in interstitial pressure. As a consequence of this interstitial pressure, the lumens of blood vessels close off and blood flow is decreased, especially to the center of the tumor.
Some preclinical data suggest that anti-VEGF therapy may actually improve, rather than inhibit, blood flow to a tumor. It's difficult to destroy blood vessels within one or two days of administering an anti-VEGF agent; however, there are several preclinical and clinical studies demonstrating that an anti-VEGF agent can alter the vascular permeability in a tumor almost immediately. This suggests that anti-VEGF therapy may affect the vasculature in some other way, possibly by inhibiting permeability. If the permeability is inhibited, interstitial pressure within a tumor may also be inhibited, and the blood vessels may open up and improve blood flow.
Anti-VEGF therapy and chemotherapy uptake by tumors
If anti-VEGF therapy improves blood flow to the tumor, the delivery of chemotherapeutic agents may also improve. The same principle holds true for radiation therapy where oxygen is required to create free radicals. Mice that are pretreated with anti-VEGF therapy and then administered chemotherapy will have an increased uptake of chemotherapy into implanted tumors.
At the present time, it is not known whether certain chemotherapeutic agents will penetrate tumors better than others. Chemotherapeutic agents that are tightly bound to albumin may have limited access to the tumor tissues, whereas chemotherapeutic agents that are free in the plasma may penetrate the tumor tissue more readily. A preclinical study found increased irinotecan uptake by tumors implanted in mice that were pretreated with anti-VEGF therapy. Uptake of oxaliplatin by tumors implanted in mice that have been pretreated with anti-VEGF therapy has not been tested, but it is certainly of interest.
Controversy exists about whether the addition of bevacizumab to chemotherapeutic agents other than irinotecan will be equally beneficial. If anti-VEGF therapy truly improves delivery of chemotherapy by increasing blood flow to a tumor, it may not matter which chemotherapeutic agent is administered.
Bevacizumab and radiation therapy
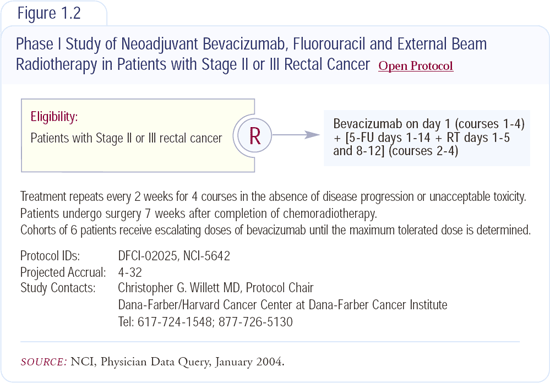
Preclinical studies have shown that anti-VEGF therapy augments the effects of radiation therapy. Chris Willett, who originally hypothesized that interstitial pressure may impede the delivery of chemotherapy or oxygen to the tumor, is investigating the influence of bevacizumab on radiation therapy in patients with rectal cancer (Figure 1.2). In that trial, patients undergo baseline studies (e.g., MRI, CT scan, biopsies and measurement of interstitial tumor pressure) and initially receive bevacizumab alone for 14 days; then the studies are repeated. Bevacizumab is then continued, and 5-FU and radiation therapy are added. The study will determine whether there's any change in flow, interstitial pressure or response rate to chemoradiation therapy associated with bevacizumab.
Bevacizumab in the management of metastatic colorectal cancer
Only Phase III clinical trials will determine whether the addition of bevacizumab to other chemotherapeutic agents will come to fruition. Many medical oncologists in academia have switched from IFL to FOLFIRI because its toxicity profile is better. It can be assumed that the addition of bevacizumab to FOLFIRI will give us the same results as the addition of bevacizumab to IFL, but this needs to be confirmed with a clinical trial.
At ASCO 2003, Rich Goldberg presented data demonstrating a median survival of 19.5 months with FOLFOX4, which is very similar to the median survival of 20.3 months seen with IFL plus bevacizumab. The choice between FOLFOX and IFL plus bevacizumab as first-line therapy will probably depend on the medical oncologist's practice and comfort level using oxaliplatin or antibodies.
Side effects associated with bevacizumab
Hypertension is the most consistent side effect and has been reported in as few as 20 percent and as many as 80 percent of patients treated with bevacizumab. It is postulated that hypertension may be a surrogate marker of biologic activity. Vascular endothelial growth factor causes induction of nitric oxide, which then causes vasodilation. Anti-VEGF therapy may block nitric oxide and produce a relative vasoconstriction. In the clinical trials, either stopping bevacizumab or increasing the dose of the patient's antihypertensive medication easily managed the hypertension. At times, another antihypertensive agent needed to be added, and it was very rare that patients were hospitalized for hypertension.
In the pivotal Phase III trial, six patients in the IFL plus bevacizumab arm and no patients in the IFL-alone arm experienced bowel perforation. The mechanism for the perforations is unknown. It is hypothesized that patients obtain such a good response to chemotherapy plus bevacizumab that their tumor, attached to the bowel, melts away. However, in reviewing the data, that does not appear to be the case. This is a toxicity that should be monitored in the future.
In some of the early bevacizumab trials, an increase in thrombosis and proteinuria was reported. However, in the large Phase III trial, there didn't appear to be any increase in thrombosis. Proteinuria was typically reversible upon cessation of the drug, but its long-term effect on the kidneys is unknown.
Select publications
|
| Dr Ellis is Professor of Surgical Oncology and Professor of Cancer Biology at the MD Anderson Cancer Center in Houston, Texas. |
|

