You
are here: Home: CCU 4 | 2003: Patrick J Flynn, MD
Edited comments by Dr Flynn
MOSAIC adjuvant trial: LV5FU2 with or without oxaliplatin
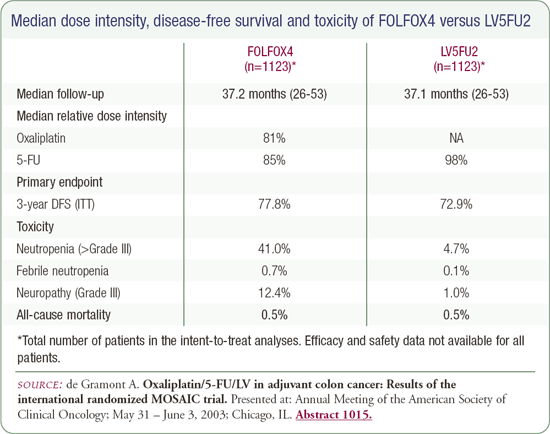
Aimery de Gramont presented data from the adjuvant trial comparing 5FU/leucovorin to 5FU/leucovorin + oxaliplatin (FOLFOX4), which resulted in a disease-free survival difference at three years. He was emphatic that the difference would not disappear with longer-term follow-up, but I've seen such differences erode in other adjuvant trials. Dr de Gramont's zeal is admirable in some ways but problematic in others. We should not be quite so dogmatic until we've had a more robust follow-up. I trust his numbers, but the duration of follow-up concerns me because it's fairly brief.
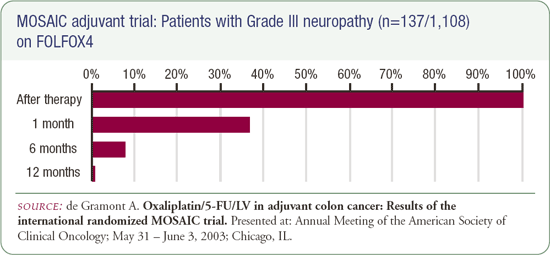
The information provided about the toxicity of 5FU/LV/oxaliplatin suggested there were a considerable number of patients treated on the FOLFOX4 regimen who developed fairly major neuropathy. They provided data that it was ameliorated over time, and the majority of patients had minimal neuropathy one year post-therapy, but I'm hesitant to recommend any therapy that causes neuropathy that interferes with my patients' daily lives until the data is published and the follow-up is longer. The data are very interesting, but I personally hope this regimen is not rapidly adopted as standard therapy at this time.

Clinical experience with oxaliplatin
Oxaliplatin is a much easier drug to use than American oncologists thought 10 years ago. The neuropathy and the cold dysesthesias aren't quite as challenging if we warn patients ahead of time to not drink really cold beverages and grab something out of the freezer with their bare hands. I have very few patients with metastatic disease who come in complaining of significant side effects. I attend to the development of neuropathy and do not let patients go forward with worsening neuropathy to the point that it interferes with ambulation or function. If they're responding, they may not be quite as honest about reporting symptoms as I'd like.
Outside of a formal clinical trial, I'm still using the original published FOLFOX4 regimen. I established relationships in my area with a couple of home-care companies that provide us with the pumps and do the authorization and the billing for the patients, so those responsibilities do not fall to my nurses. It's a very simple therapy to administer now that the orchestration of the people in charge of the pump is routine.
In talking with my colleagues, I believe there is more concern about use of pumps than there ought to be. Pumps are not that difficult to manage, and patient acceptance is not problematic. Nonetheless, there's still a significant tendency for American oncologists to resist the use of ambulatory pumps.
Prolonged survival with the addition of bevacizumab to IFL in metastatic disease
A trial presented at ASCO 2003 evaluated IFL - the Saltz regimen - with and without bevacizumab. Originally, there was an additional arm combining 5FU/leucovorin plus bevacizumab, because at the time the trial opened it had not been established that irinotecan improved response rates in metastatic colorectal cancer.
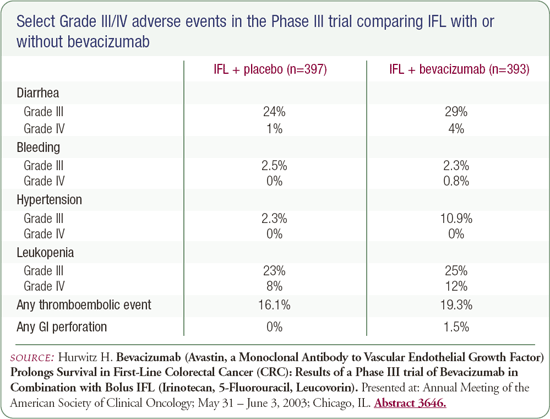
The addition of bevacizumab - a monoclonal antibody to vascular endothelial growth factor - involved more patient visits but had relatively minimal toxicity. The 4.7-month improvement in overall survival with bevacizumab is a solid breakthrough in the treatment of colorectal cancer.
In a nonprotocol setting, I would use bevacizumab with the Saltz regimen because that's where we have data. I'm very hopeful that in the very near future we're going to have data combining bevacizumab with other regimens using oxaliplatin.
The FOLFOX4 regimen in the North Central Cancer Treatment Group protocol clearly had a better response rate and duration of response when it was compared to IFL, but IFL plus bevacizumab resulted in overall survival very similar to FOLFOX4. We don't know if adding bevacizumab to FOLFOX4 results in additional benefit, and we don't know the nature of the toxicities. In a nonprotocol situation, I would not utilize bevacizumab in combination with FOLFOX4.
Deciding between FOLFOX4 and IFL plus bevacizumab will require a lengthy discussion with patients, and I don't know which option patients will choose. With bevacizumab, patients have to be willing to be on pumps. This regimen is something new that's a challenge to talk to patients about, but it's a great option to have. We have two regimens that produced a major improvement in overall survival. Those are the kinds of problems oncologists would like to have more often.
Tolerability of bevacizumab in combination with IFL
The most noticeable side effect associated with bevacizumab was that about one-quarter of patients developed problems with hypertension, but this was relatively simple to treat. Usually, something as simple as a diuretic, like hydrochlorothiazide, could be used to treat the hypertension. It wasn't a significant problem to manage.
Combining bevacizumab with IFL raised concerns about whether there'd be many patients with renal injury resulting in proteinuria. In my experience, that's pretty rare in patients treated with bevacizumab. Another concern was whether bevacizumab would cause more problems with bleeding or thrombosis, and there really wasn't a demonstrable difference in the two arms.
A very small number of patients had GI perforations, and that will have to be addressed in larger studies. Gastrointestinal perforations occurred in about one to two percent of patients, and it's something that we need to be aware of when it's used more broadly in clinical practice.
Select publications
|

