You
are here: Home: CCU 4 | 2003: Robert B Diasio, MD
Edited comments by Dr Diasio
MOSAIC trial
The MOSAIC adjuvant trial compared the de Gramont regimen (infusional 5-FU and leucovorin) to the FOLFOX4 regimen (infusional 5-FU, leucovorin and oxaliplatin). Approximately 1,100 patients per arm were enrolled in the trial; about one-third had Stage II disease and two-thirds had Stage III disease.
After three and one-half years, there was a relative disease-free survival risk-reduction of 23 percent associated with FOLFOX4. Some concerns were raised about using disease-free survival as an endpoint because most United States adjuvant trials have utilized overall survival, which requires a longer time to obtain meaningful data. However, the disease-free survival from the MOSAIC trial was comparable to the disease-free survival in some of the earlier United States adjuvant trials.
This trial represents the first evidence that the addition of another agent to 5-FU and leucovorin has a benefit in the adjuvant setting. As with almost all of the other adjuvant studies conducted, the benefit is in patients with Stage III disease, not Stage II disease. Tantalizing data, however, suggests that patients with Stage II disease also benefited, but it was not a statistically significant benefit.
FOLFOX4 was very tolerable. The major problem was neurotoxicity - 12 percent of the patients receiving FOLFOX4 experienced Grade III neurotoxicity (e.g., functional impairment in manual dexterity). It must be emphasized, however, that the neurotoxicity was rapidly reversible.
After 12 months, only one percent of the patients had neurotoxicity. A strategy to manage oxaliplatin-associated neurotoxicity - involving the administration of calcium and magnesium before and after oxaliplatin - was presented at ASCO 2002.
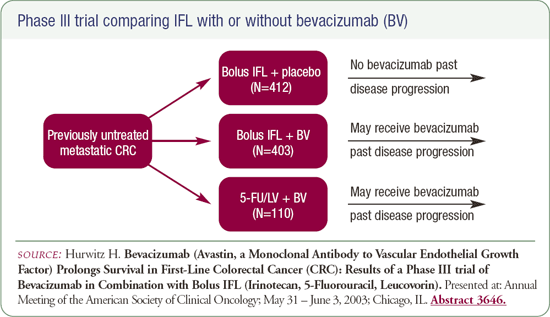
Bevacizumab in combination with IFL in patients with metastatic disease
Results of the Phase III study evaluating the combination of bevacizumab and IFL were presented as a late-breaking abstract at the ASCO 2003 GI Plenary Session. In patients with metastatic disease, a 4.7-month increase in median overall survival was observed when bevacizumab was added to IFL.
The toxicities associated with bevacizumab were minimal, mainly mild hypertension. In all cases, the hypertension was managed with antihypertensive drugs that were administered on an outpatient basis. Another toxicity we need to be aware of is perforation of the GI tract.
Six patients out of the 400 treated with bevacizumab were noted to have evidence of perforations. No similar events occurred in the patients treated with IFL and placebo. Overall, the toxicity profile was not a major limitation to the use of bevacizumab - a very impressive agent that provides a marked benefit when administered with IFL.
Mechanism of action of bevacizumab
Bevacizumab is a chimerized antibody - more than 90 percent of it is humanized and a relatively small part is murine. This particular antibody is capable of complexing with the vascular endothelial growth factor (VEGF) released by the tumor.
A number of different factors upstream of the tumor are thought to stimulate VEGF. Following its release, VEGF acts downstream on receptors within endothelial cells in the blood vessels; this potentially increases vascularization of the area within the tumor and influences metastases. Bevacizumab couples with the released VEGF and prevents it from working at the endothelial sites.

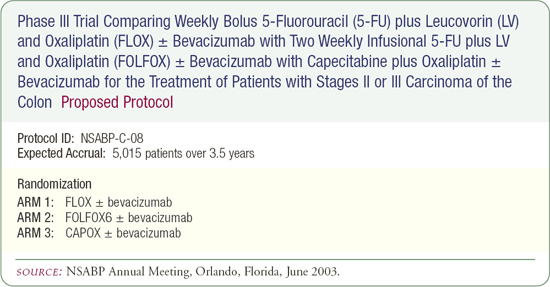
NSABP-C0-8: Proposed adjuvant trial
The planned adjuvant bevacizumab trial (NSABP-C-08) has gone through several iterations, and questions remain about the design. One of the current considerations is to compare the FLOX regimen to a FOLFOX regimen, although it's not clear at this point which of the FOLFOX regimens should be compared.
The MOSAIC trial evaluated FOLFOX4, but most United States cooperative group studies now incorporate FOLFOX6 and even FOLFOX7. There's also interest in evaluating the CAPOX regimen, a combination of capecitabine and oxaliplatin. The proposed NSABP-C0-8 may compare the Roswell Park regimen of 5-FU administration to a FOLFOX regimen and to CAPOX, each administered with or without bevacizumab.
First-line therapy for patients with metastatic colorectal cancer
When treating patients with advanced colorectal cancer, we have three very active drugs: 5-FU, irinotecan and oxaliplatin. The comparative studies, however, are difficult to evaluate because N9741 compared the Saltz regimen (bolus 5-FU) to FOLFOX (infusional 5-FU). Although we are comparing apples and oranges in N9741, I think oxaliplatin still shows some improvement. My bias is to treat patients with a FOLFOX regimen first-line.
Historically, most of us have used FOLFOX4, but the convenience of FOLFOX6 and FOLFOX7 makes them much more appealing. In the university setting, we are using FOLFOX6 at the moment. We were impressed with the data on FOLFOX7 presented at ASCO 2003, which involves a higher oxaliplatin dose and, if necessary, stopping the regimen and using intermittent therapy -something that goes against our traditional teaching in oncology. However, as reported at ASCO 2003, FOLFOX7 can be administered intermittently with very positive results compared to FOLFOX4 administered continuously.
To summarize, I would use an oxaliplatin-containing regimen as first-line therapy in a patient with metastatic colorectal cancer. Since I'm more familiar with FOLFOX6, I would probably use that regimen. Then, if the patient's disease progressed in the future, I would consider irinotecan.
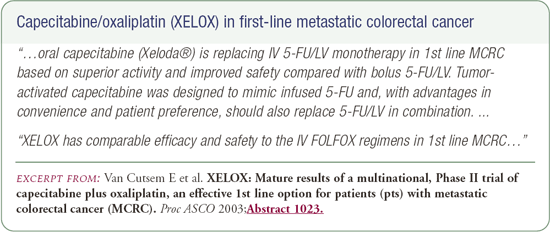
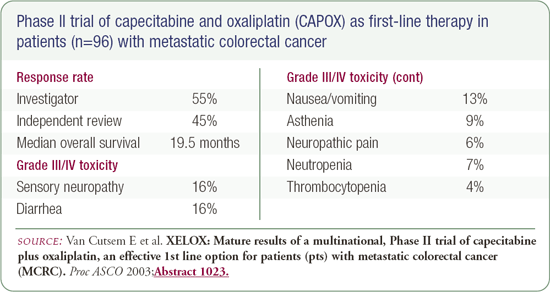
CAPOX in patients with metastatic disease
At ASCO 2003, Van Cutsem reported on a Phase II trial evaluating the combination of capecitabine and oxaliplatin (CAPOX). This small trial (n=96) demonstrated an overall median survival of 19.5 months. A number of trials that will be conducted by the United States cooperative groups (i.e., NSABP, SWOG, ECOG) will evaluate the CAPOX regimen because it avoids the problem American oncologists have with infusional 5-FU.
CAPOX is a worthwhile alternative that should be discussed with patients. Some oncologists don't have an infusional service available for the administration of infusional 5-FU, and they ask what they should do. I initially say, "We have very exciting data with CAPOX; I don't recommend it up front, and there are caveats to be emphasized in terms of diarrhea when using capecitabine." I have treated patients with CAPOX, and I have recommended that oncologists use it in select situations. It's a much easier way to administer 5-FU, and the data on overall survival is very impressive from the small Phase II study.
Select publications
|

