You
are here: Home: CCU 4 | 2003: Charles D Blanke, MD, FACP
Edited comments by Dr Blanke
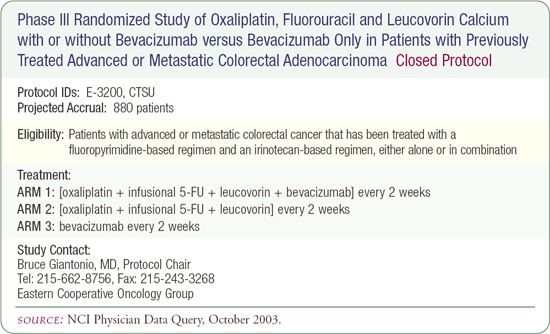
SWOG-S0303: Phase III trial in patients with locally advanced metastatic or recurrent colorectal cancer
This trial will address two important questions. First, is capecitabine and oxaliplatin equivalent to infusional 5-FU and oxaliplatin? Oxaliplatin has moved into the front-line treatment of patients with metastatic colorectal cancer. We are interested in exploring whether the infusional 5-FU component of the regimen is necessary or whether it can be substituted by an oral fluoropyrimidine, like capecitabine.
Secondly, can we improve survival with the addition of bevacizumab? Bevacizumab was recently shown to improve the median survival associated with chemotherapy by about five months. Therefore, we will also randomly assign patients to bevacizumab or placebo. I think capecitabine and oxaliplatin will be at least equivalent to infusional 5-FU and oxaliplatin. We're hoping that bevacizumab improves median survival substantially.
Rationale for SWOG-S0303
When a survival advantage was demonstrated with the combination of 5-FU, leucovorin and irinotecan, we moved away from standard 5-FU to triple therapy. For several years, we used irinotecan with bolus 5-FU and leucovorin (IFL). It was the best therapy we had to offer, and it was better than standard 5-FU alone. Rich Goldberg headed up N9741, the trial that compared bolus IFL to infusional 5-FU, leucovorin and oxaliplatin (FOLFOX). FOLFOX proved to be markedly better and less toxic. Whether the infusional 5-FU or oxaliplatin was responsible for the difference is not clear; however, FOLFOX was clearly better, and we chose it as the control arm for SWOG-S0303.
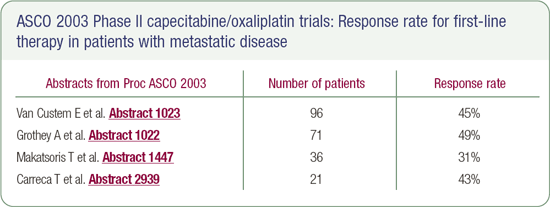
Since capecitabine is as effective - perhaps a little more effective - and less toxic than 5-FU alone, it seemed like a logical combination with oxaliplatin. In addition, Phase II data indicate that capecitabine in combination with oxaliplatin has a very high response rate and a favorable median survival.

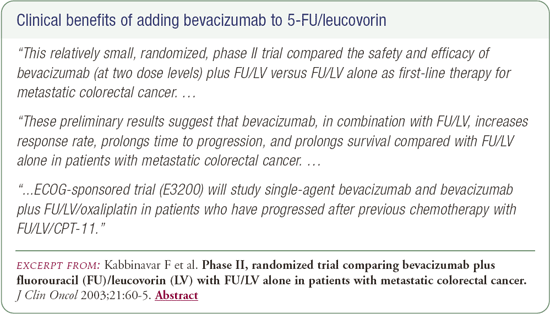
Bevacizumab in the nonprotocol setting
If bevacizumab were available today, I would definitely use it as second-line therapy in a nonprotocol setting. FOLFOX tends to be my first-line regimen because I have the most experience and excellent results with it. If there were no trial to offer a patient with disease that had failed FOLFOX, I would probably recommend 5-FU and irinotecan with bevacizumab. The question of whether to use bevacizumab as first-line therapy is still unanswered, although there are other experts who would use it right now.
If a patient had received first-line irinotecan, I would consider an oxaliplatin-based regimen plus bevacizumab in a nonprotocol situation, but we don't have data yet. From the ECOG trial evaluating bevacizumab and oxaliplatin as second-line therapy, we know that it's safe, and the efficacy data should be reported soon.
Celecoxib and chemotherapy-related toxicity
We recently reported preliminary results from a Phase II trial evaluating IFL in combination with celecoxib. Interestingly, there may be a dramatic reduction in chemotherapy-related toxicity. The incidence of diarrhea - particularly severe diarrhea - decreased, and there may also be a reduction in myelosuppression. It's too early to tell whether there will be an improvement in efficacy.
Dr Edward Lin reported that celecoxib might improve capecitabine-associated toxicity. If celecoxib could prevent the hand-foot syndrome, even if it didn't improve efficacy, it would be a worthwhile addition. Other preliminary data indicate that celecoxib may affect oxaliplatin-mediated neurotoxicity, which would be a huge breakthrough. We're discussing whether we should evaluate that in a formal trial.
Approaches to adjuvant therapy
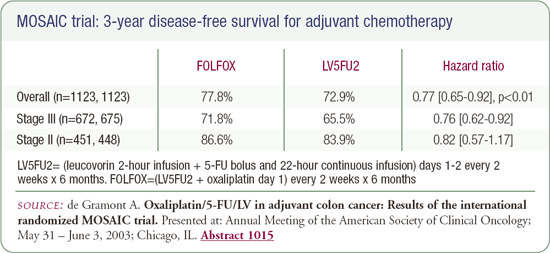
Dr de Gramont's presentation at ASCO demonstrated an advantage in disease-free survival for adjuvant FOLFOX. It was a modest improvement of roughly five percent, but it's not a particularly toxic regimen, so I think it was enough to offer to patients. In 2003, it is reasonable to offer adjuvant FOLFOX to patients with Stage III disease. It is also reasonable to discuss whether patients with Stage II disease should receive chemotherapy and, if they are going to be treated, it is reasonable to consider oxaliplatin, although that would be aggressive.
Data will soon emerge regarding the adjuvant irinotecan trial. The preliminary data indicate that that trial is negative, which is difficult to explain. Based on the current data, I would not offer irinotecan in the adjuvant setting, but I definitely would like to see additional follow-up from the CALGB trial.
Current research in pancreatic cancer
Pancreatic cancer remains the malignant neoplasm with the shortest five-year survival of the gastrointestinal tumors. Essentially, we don't have good treatments. Standard chemotherapy offers about a five percent remission rate and a few weeks of improvement in median survival.
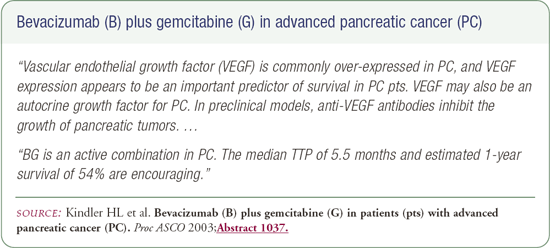
This is the era of doublet therapy. We have gemcitabine, which I use all the time off protocol. It's being evaluated in combination with a number of other chemotherapeutic agents, but also with some targeted therapies. In the chemotherapeutic arena, there is emerging data on its use with the platinums -particularly cisplatin and, even more promising, oxaliplatin.
Finally, there is emerging data for gemcitabine in combination with either bevacizumab or cetuximab (C225). There was a small trial that combined bevacizumab with gemcitabine, which had some nice biologic correlates. There was presumed clinical benefit, and the regimen was actually very well-tolerated.
Gastrointestinal stromal tumor (GIST)
GISTs weren't recognized six or seven years ago, but now they are one of the most common soft-tissue sarcomas. Somewhere between 5,000 and 10,000 GISTs are diagnosed in the United States every year. The usual clinical presentation is GI bleeding. These tumors become enormously large, auto-ulcerate and outgrow their blood supply. They do not respond to standard cytotoxic chemotherapy, including agents that are active in other sarcomas (i.e., doxorubicin, ifosfamide or gemcitabine).
Studies were conducted at Oregon Health & Science University in patients with chronic myeloid leukemia (CML) utilizing the drug imatinib mesylate. It turns out that patients with GISTs have a genetic defect, which leads to the activation of a gene and subsequent protein production that is very similar to the one seen in CML.
Basic science research indicated that the KIT oncoprotein could be inhibited by imatinib. Therefore, we conducted a Phase II trial with imatinib and found a 65 percent response rate with GIST. Three years later, more than half of the patients in that original trial are alive and well, although some patients relapsed or progressed in about 15 to 18 months.

Select publications
|

