
Select Excerpts from the Discussion
Tracks 46, 48
 DR LOVE:
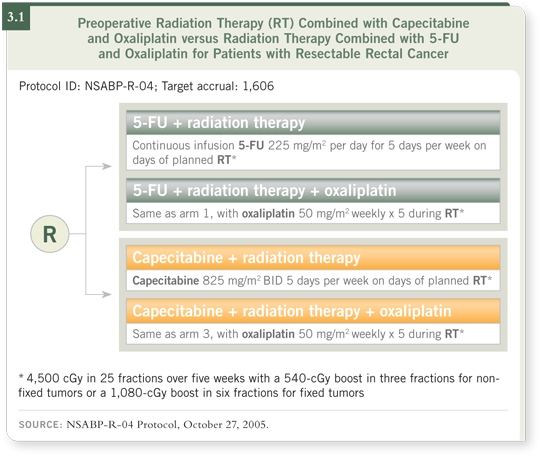
DR LOVE: Dan, can you discuss NSABP-R-04 (3.1) and how you feel
about the use of oxaliplatin as neoadjuvant therapy?
 DR HALLER: I wear two different hats: one as a person who conducted a
Phase I/II trial in this area and uses it off protocol and another as the GI
Intergroup co-chair of NSABP-R-04, specifically addressing this question.
The primary comparison in NSABP-R-04 was capecitabine versus infusional
5-FU. It became a pedestrian question because many physicians had already
diverted to using capecitabine. However, it remains an important issue because you have to lower the dose of capecitabine from the “standard systemic dose.”
You need to ensure you’re maintaining — for a patient being treated with
curative intent — the same rates obtained with infusional 5-FU.
DR HALLER: I wear two different hats: one as a person who conducted a
Phase I/II trial in this area and uses it off protocol and another as the GI
Intergroup co-chair of NSABP-R-04, specifically addressing this question.
The primary comparison in NSABP-R-04 was capecitabine versus infusional
5-FU. It became a pedestrian question because many physicians had already
diverted to using capecitabine. However, it remains an important issue because you have to lower the dose of capecitabine from the “standard systemic dose.”
You need to ensure you’re maintaining — for a patient being treated with
curative intent — the same rates obtained with infusional 5-FU.
 DR LOVE: What is the dosing schedule for capecitabine in NSABP-R-04?
DR LOVE: What is the dosing schedule for capecitabine in NSABP-R-04?
 DR HALLER: It’s 825 mg/m2 twice a day on Monday through Friday. As the
study was just about to be launched, the whole oxaliplatin issue came along.
Many of us thought the trial would be better as a two-by-two design asking
two important questions. We will have the definitive answer from NSABPR-
04, but I’m guessing that the oxaliplatin combinations will have better pCR
rates. Whether that translates into better long-term outcomes — including
acute and late toxicities, sphincter preservation, type of surgery and overall
survival — is where NSABP-R-04 comes into play.
DR HALLER: It’s 825 mg/m2 twice a day on Monday through Friday. As the
study was just about to be launched, the whole oxaliplatin issue came along.
Many of us thought the trial would be better as a two-by-two design asking
two important questions. We will have the definitive answer from NSABPR-
04, but I’m guessing that the oxaliplatin combinations will have better pCR
rates. Whether that translates into better long-term outcomes — including
acute and late toxicities, sphincter preservation, type of surgery and overall
survival — is where NSABP-R-04 comes into play.
 DR LOVE: What do we know about the safety and tolerability of adding oxaliplatin
to a fluoropyrimidine in the neoadjuvant setting?
DR LOVE: What do we know about the safety and tolerability of adding oxaliplatin
to a fluoropyrimidine in the neoadjuvant setting?
 DR HALLER: In most of the studies, more diarrhea occurred with the weekly
regimen, which is why more doses were dropped. Patients received more
oxaliplatin administered less frequently because they didn’t face dose reductions
and delays. In rectal cancer, it’s important that you not interfere with the
radiation therapist administering treatment at the standard dose and schedule.
DR HALLER: In most of the studies, more diarrhea occurred with the weekly
regimen, which is why more doses were dropped. Patients received more
oxaliplatin administered less frequently because they didn’t face dose reductions
and delays. In rectal cancer, it’s important that you not interfere with the
radiation therapist administering treatment at the standard dose and schedule.
Select Publications

