
Select Excerpts from the Discussion
Tracks 7-8, 17
 DR LOVE:
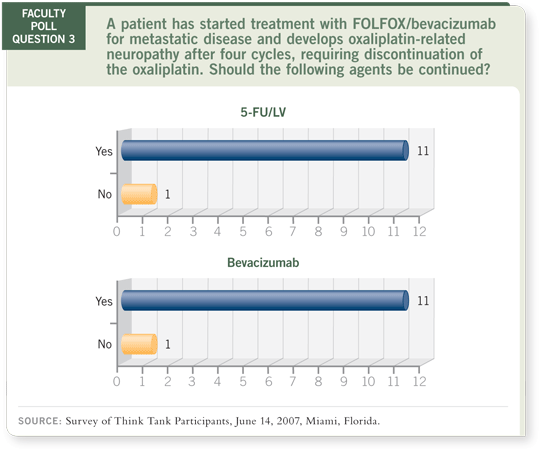
DR LOVE: Neal, assume a patient has started on FOLFOX/bevacizumab
for metastatic disease and after four cycles develops oxaliplatin-related
neuropathy, which requires discontinuation of the oxaliplatin. Our faculty
poll showed that 11 of 12 faculty members said that both 5-FU and
bevacizumab should be continued. Any thoughts?
 DR MEROPOL: This is pretty straightforward. You’re doing well on therapy
and have a reason to stop oxaliplatin — it’s not a reason to stop the other
agents. Good data from the early studies with 5-FU/bevacizumab suggest it’s better than 5-FU alone (Kabbinavar 2003), so I believe that is a
reasonable approach.
DR MEROPOL: This is pretty straightforward. You’re doing well on therapy
and have a reason to stop oxaliplatin — it’s not a reason to stop the other
agents. Good data from the early studies with 5-FU/bevacizumab suggest it’s better than 5-FU alone (Kabbinavar 2003), so I believe that is a
reasonable approach.
When I see the point estimates for survival showing a seven-month difference
between total cessation of therapy and continuation of some therapy during
the holiday (Maindrault-Goebel 2007), I find that to be striking in terms of its
magnitude. I don’t believe anybody around this table would predict a seven-month
difference in survival. If the point estimate is fairly accurate, that’s a
huge magnitude of difference — bigger than the difference between any two
treatment arms I’ve seen since I’ve been a medical oncologist.
 DR LOVE: In terms of quality of life, how much of an improvement do you
see with an OPTIMOX-like strategy?
DR LOVE: In terms of quality of life, how much of an improvement do you
see with an OPTIMOX-like strategy?
 DR GROTHEY: Patients benefit from discontinuing oxaliplatin at some
point and continuing only a fluoropyrimidine and a biologic agent. It’s not
only neurotoxicity. Oxaliplatin is associated with other toxicities. It increases
diarrhea, fatigue, nausea and vomiting. Platinum drugs are known to cause
fatigue. So when not receiving oxaliplatin, patients feel better.
DR GROTHEY: Patients benefit from discontinuing oxaliplatin at some
point and continuing only a fluoropyrimidine and a biologic agent. It’s not
only neurotoxicity. Oxaliplatin is associated with other toxicities. It increases
diarrhea, fatigue, nausea and vomiting. Platinum drugs are known to cause
fatigue. So when not receiving oxaliplatin, patients feel better.
Irinotecan is similar. I have patients with more nausea and vomiting from
irinotecan than from oxaliplatin. Fatigue is probably not as pronounced as it is
with platinum drugs, in general. The idea of an OPTIMOX-like strategy for
FOLFIRI makes a lot of sense.
Tracks 4-5
 DR LOVE:
DR LOVE: Let’s talk about the iBET trial (SWOG-S0600), which just
opened in June. What’s the rationale behind it?
 DR GROTHEY: Based on the BRiTE registry data (Grothey 2007) and other
data from clinical practice, there is a hypothesis that continuing bevacizumab
beyond the first tumor progression would make sense and even influence
outcomes.
DR GROTHEY: Based on the BRiTE registry data (Grothey 2007) and other
data from clinical practice, there is a hypothesis that continuing bevacizumab
beyond the first tumor progression would make sense and even influence
outcomes.
This is not yet proven. It’s a hypothesis and is supported by some
biologic models. Many oncologists are doing this in clinical practice, and it
is an ideal situation for running a clinical trial to answer the question, is it
worthwhile to continue bevacizumab beyond progression?
 DR LOVE: Of course, this is the study that was never conducted with
trastuzumab in breast cancer.
DR LOVE: Of course, this is the study that was never conducted with
trastuzumab in breast cancer.
 DR GROTHEY: Yes. The implications for oncology are larger with
bevacizumab or anti-VEGF therapy than with trastuzumab because
trastuzumab is only suitable for 20 to 25 percent of patients with breast cancer.
With bevacizumab and other anti-VEGF agents, we’re talking about all
patients with a lot of different cancers.
DR GROTHEY: Yes. The implications for oncology are larger with
bevacizumab or anti-VEGF therapy than with trastuzumab because
trastuzumab is only suitable for 20 to 25 percent of patients with breast cancer.
With bevacizumab and other anti-VEGF agents, we’re talking about all
patients with a lot of different cancers.
 DR LOVE: What are the eligibility criteria for the iBET study?
DR LOVE: What are the eligibility criteria for the iBET study?
 DR GROTHEY: The iBET study will enroll patients who have had documented progression on first-line therapy with an oxaliplatin- and bevacizumab-based
regimen. Any kind of oxaliplatin-based therapy is allowed — FOLFOX4,
FOLFOX6, modified FOLFOX7, CAPOX or an OPTIMOX-like regimen.
DR GROTHEY: The iBET study will enroll patients who have had documented progression on first-line therapy with an oxaliplatin- and bevacizumab-based
regimen. Any kind of oxaliplatin-based therapy is allowed — FOLFOX4,
FOLFOX6, modified FOLFOX7, CAPOX or an OPTIMOX-like regimen.

The trial has a three-arm randomization. Patients on all three arms will
receive an irinotecan- and cetuximab-based regimen (Grothey 2007). Two of
the arms will carry over bevacizumab, one at a low dose of 5 mg/kg and the
other at a high dose of 10 mg/kg (2.1). So this trial will also try to answer the
question, what’s the appropriate dose of bevacizumab as second-line therapy?
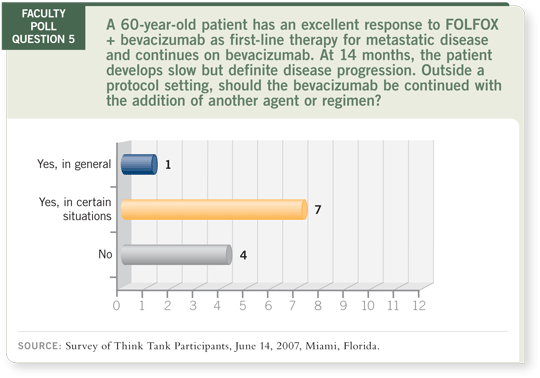
 DR LOVE: Axel, what do you expect to see in the iBET trial?
DR LOVE: Axel, what do you expect to see in the iBET trial?
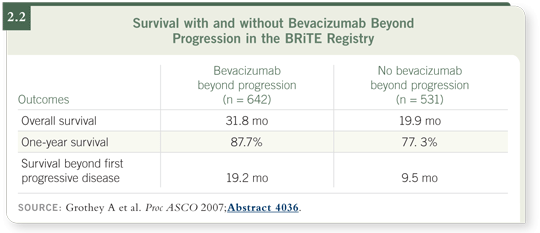
 DR GROTHEY: Based on the analysis of the BRiTE registry (Grothey 2007;
[2.2]), I believe the trial will be positive for the continuation of bevacizumab
on progression.
DR GROTHEY: Based on the analysis of the BRiTE registry (Grothey 2007;
[2.2]), I believe the trial will be positive for the continuation of bevacizumab
on progression.
 DR ELLIS: I don’t expect to see a difference between the arms, and in the
second-line setting, the dose will probably not make much of a difference. I
believe cetuximab is a good choice as second-line therapy. I believe investigators
will find minimal differences among the three arms.
DR ELLIS: I don’t expect to see a difference between the arms, and in the
second-line setting, the dose will probably not make much of a difference. I
believe cetuximab is a good choice as second-line therapy. I believe investigators
will find minimal differences among the three arms.
Tracks 10-12
 DR LOVE:
DR LOVE: Herb, is there any reason to think there’s an advantage to
using bevacizumab with an irinotecan-containing regimen, such as IFL,
compared to FOLFOX?
 DR HURWITZ: The take-home message from the NO16966 study is that
FOLFOX and CAPOX are essentially equivalent (Cassidy 2007). Second, the
addition of bevacizumab to first-line therapy with oxaliplatin is beneficial
(Saltz 2007a).
DR HURWITZ: The take-home message from the NO16966 study is that
FOLFOX and CAPOX are essentially equivalent (Cassidy 2007). Second, the
addition of bevacizumab to first-line therapy with oxaliplatin is beneficial
(Saltz 2007a).
The third message is that cross-study comparisons are problematic, and much
of the size of the treatment effect, which we often discuss as highly relevant,
can be related to artifacts of trial design or imbalances in patient characteristics,
such as nuances in how progression was defined, the fraction of patients
with PS 2, age and gender.
Fourth, I would regard this trial as one of many aggregate studies of
bevacizumab in colon cancer, which is consistent with a treatment benefit.
With all of those caveats, I don’t believe these data support any conclusion
related to special interactions between bevacizumab and irinotecan versus
oxaliplatin.
 DR LOVE: Lee, there is a lot of interest in new biologic agents for the treatment of metastatic colon cancer. Can you discuss the multikinase inhibitor vandetanib?
DR LOVE: Lee, there is a lot of interest in new biologic agents for the treatment of metastatic colon cancer. Can you discuss the multikinase inhibitor vandetanib?
 DR ELLIS: Vandetanib, or AZD6474, targets multiple tyrosine kinases,
primarily VEGF receptors, and EGF receptors. It’s important to point out that
what you gain on one hand, you lose on the other. As EGFR is increasingly
inhibited, the potency toward inhibiting VEGFR is lessened.
DR ELLIS: Vandetanib, or AZD6474, targets multiple tyrosine kinases,
primarily VEGF receptors, and EGF receptors. It’s important to point out that
what you gain on one hand, you lose on the other. As EGFR is increasingly
inhibited, the potency toward inhibiting VEGFR is lessened.
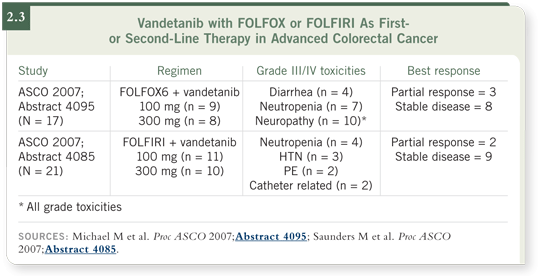
At ASCO this year, two dose-escalation studies were presented — one with
FOLFOX (Michael 2007) and one with FOLFIRI (Saunders 2007). Treatment
with vandetanib was initiated at 100 milligrams. If fewer than two out of six
patients experienced dose-limiting toxicities, then the dose was increased to
300 milligrams a day (2.3).
The most common side effect that occurred with FOLFOX was neuropathy in
10 out of 17 patients. Seven patients had neutropenia, and four had diarrhea.
In the study of FOLFIRI with vandetanib, four patients had neutropenia,
three had hypertension, two experienced pulmonary emboli and a few had
catheter-related events.
The best response was similar between the two studies. In the study evaluating
FOLFOX with vandetanib, there were three partial responses in 17 patients
and eight patients with stable disease at two months (Michael 2007).
With the addition of vandetanib to FOLFIRI, there were two partial responses
out of 21 patients, and nine patients had stable disease (Saunders 2007).
Overall, vandetanib is relatively safe, but we need to accrue more patients and
evaluate this in a separate setting.
Track 22
 DR LOVE:
DR LOVE: Let’s talk about treatment of metastatic disease for cure. Bob,
do you believe that more patients should be considered for potentially
curative resection of liver metastases?
 DR WOLFF: My view, when I see patients referred from outside, is that these
practices are not being followed with discipline. No plan of action may be in
place when liver metastases are found.
DR WOLFF: My view, when I see patients referred from outside, is that these
practices are not being followed with discipline. No plan of action may be in
place when liver metastases are found.
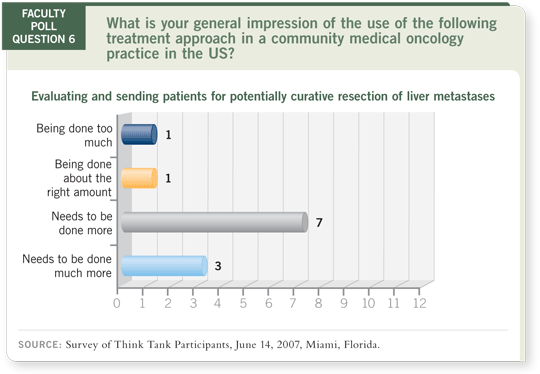
Sometimes physicians send their patients immediately to a surgeon, and
sometimes they initiate therapy. No algorithm is apparent in terms of how this
situation is approached.
The other problem I notice is that when a hepatic surgeon is not available, the
thinking is that radiofrequency ablation is equivalent. So when good hepatic
surgery is not available, some oncologists say, “We didn’t have a hepatic
surgeon, so we went for radiofrequency ablation and used chemotherapy on
the back end.”
Track 25
 DR LOVE:
DR LOVE: What about preoperative systemic therapy before surgery to
remove liver metastases?
 DR HALLER: For a patient presenting with potentially resectable hepatic
metastases, I send the patient to surgery first and then consider adjuvant therapy.
DR HALLER: For a patient presenting with potentially resectable hepatic
metastases, I send the patient to surgery first and then consider adjuvant therapy.
 DR HOCHSTER: I believe at this point surgery alone should not be acceptable.
Whether systemic therapy is used preoperatively or postoperatively is still a
valid question.
DR HOCHSTER: I believe at this point surgery alone should not be acceptable.
Whether systemic therapy is used preoperatively or postoperatively is still a
valid question.
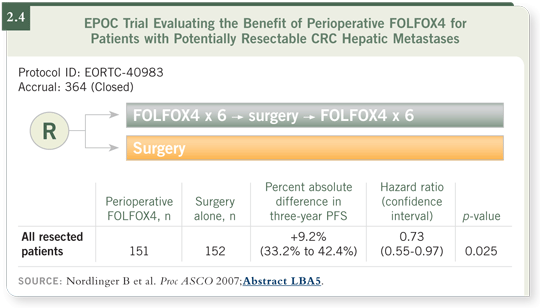
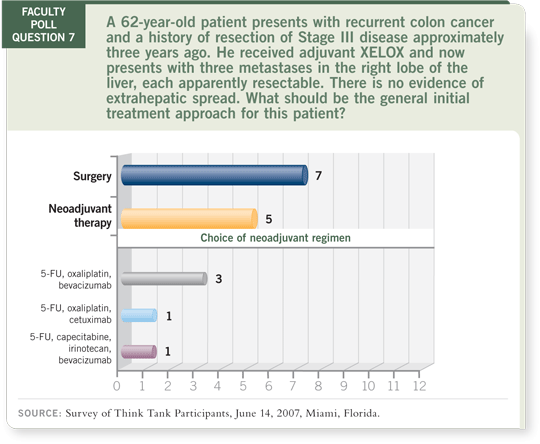
 DR WOLMARK: Even if we assume that the Nordlinger study was unequivocally
positive (Nordlinger 2007; [2.4]), it tells us absolutely nothing about whether
preoperative chemotherapy is useful. That variable was completely untested. So I consider the seven votes in our faculty poll for surgery as a lower response than it
ought to be.
DR WOLMARK: Even if we assume that the Nordlinger study was unequivocally
positive (Nordlinger 2007; [2.4]), it tells us absolutely nothing about whether
preoperative chemotherapy is useful. That variable was completely untested. So I consider the seven votes in our faculty poll for surgery as a lower response than it
ought to be.
 DR GROTHEY: In that trial, chemotherapy was useful in the context of surgery.
The trial doesn’t tell us anything about neoadjuvant versus postoperative. I do
believe that right now, patients with resected Stage IV disease should receive
some form of chemotherapy in the context of surgery (Nordlinger 2007).
DR GROTHEY: In that trial, chemotherapy was useful in the context of surgery.
The trial doesn’t tell us anything about neoadjuvant versus postoperative. I do
believe that right now, patients with resected Stage IV disease should receive
some form of chemotherapy in the context of surgery (Nordlinger 2007).
Select Publications

