
 |
|||||||

| Tracks 1-15 | ||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: Would you discuss the background and design of the NSABPR-04 trial evaluating neoadjuvant treatment for rectal cancer?
DR LOVE: Would you discuss the background and design of the NSABPR-04 trial evaluating neoadjuvant treatment for rectal cancer?
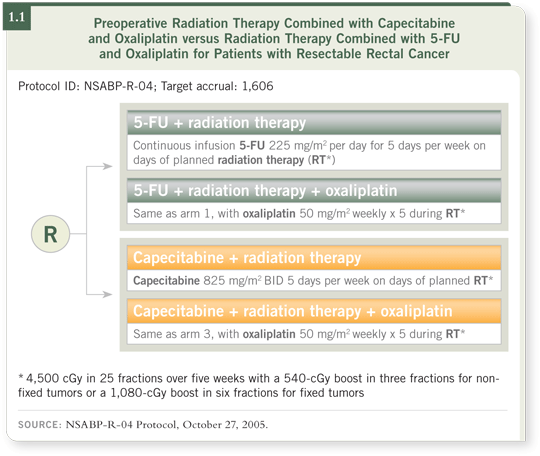
![]() DR O’CONNELL : The NSABP-R-04 study is evaluating different methods of
combined-modality neoadjuvant treatment using continuous infusion 5-FU combined with radiation therapy preoperatively as the standard, and it’s investigating
whether capecitabine would be equally effective as 5-FU in a noninferiority
analysis.
DR O’CONNELL : The NSABP-R-04 study is evaluating different methods of
combined-modality neoadjuvant treatment using continuous infusion 5-FU combined with radiation therapy preoperatively as the standard, and it’s investigating
whether capecitabine would be equally effective as 5-FU in a noninferiority
analysis.
The trial is also evaluating whether the addition of oxaliplatin in the preoperative setting might increase the pathological response rates and improve long term local control (1.1).
![]() DR LOVE: Do you have any predictions about what we will see with the study?
DR LOVE: Do you have any predictions about what we will see with the study?
![]() DR O’CONNELL : We hope it will be possible to use an oral agent along with
radiation therapy to be equally effective without the need for a central venous
catheter and the ambulatory infusion pump.
DR O’CONNELL : We hope it will be possible to use an oral agent along with
radiation therapy to be equally effective without the need for a central venous
catheter and the ambulatory infusion pump.
With the known radiation-sensitizing effect of oxaliplatin and its activity combined with fluorinated pyrimidines in colorectal cancer, I believe it’s reasonable that the addition of oxaliplatin might improve local control. Obviously we have to run the trial and examine the results.
Track 4
![]() DR LOVE: What are the key research issues surrounding adjuvant systemic
therapy?
DR LOVE: What are the key research issues surrounding adjuvant systemic
therapy?
![]() DR O’CONNELL : The major question is whether the addition of targeted
agents — bevacizumab and the anti-endothelial growth factor receptor
(EGFR) monoclonal antibodies cetuximab or panitumumab — to chemotherapy
will improve the long-term therapeutic outcome. I believe we will
have a definitive answer about whether bevacizumab adds to the adjuvant
treatment of Stage II and Stage III colon cancer within the next two or
three years.
DR O’CONNELL : The major question is whether the addition of targeted
agents — bevacizumab and the anti-endothelial growth factor receptor
(EGFR) monoclonal antibodies cetuximab or panitumumab — to chemotherapy
will improve the long-term therapeutic outcome. I believe we will
have a definitive answer about whether bevacizumab adds to the adjuvant
treatment of Stage II and Stage III colon cancer within the next two or
three years.
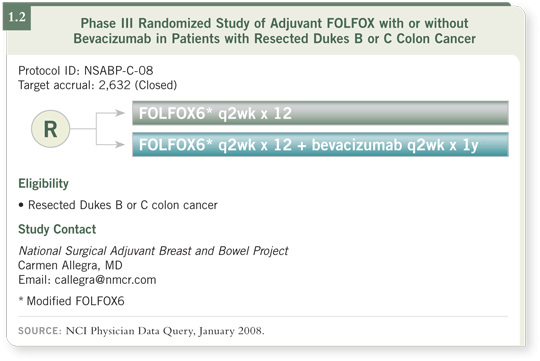
The NSABP-C-08 study has randomly assigned about 2,700 patients with Stage II and III colon cancer to receive a modified FOLFOX6 regimen for six months, as the control, or the same treatment with bevacizumab for a year (1.2). The trial completed accrual a year ago. The primary endpoint is three-year disease free survival. Of course, if there’s a striking benefit, we might hear that early. But by 2009 at the latest we should have a definitive analysis of that trial.
![]() DR LOVE: Where are we right now with safety data from that trial?
DR LOVE: Where are we right now with safety data from that trial?
![]() DR O’CONNELL : We will report this at ASCO 2008. We did not see an increase
in GI perforations among these patients in the adjuvant setting, who are different
from patients with advanced disease. Only two or three perforations were recorded
in the entire study.
DR O’CONNELL : We will report this at ASCO 2008. We did not see an increase
in GI perforations among these patients in the adjuvant setting, who are different
from patients with advanced disease. Only two or three perforations were recorded
in the entire study.
Second, we did not see an increase in arterial thrombotic strokes or heart attacks in this group of patients, who are more medically fit and younger than patients with advanced disease.
We did see a slight increase in wound infections, which was statistically significant and should be taken into consideration. It was of modest degree, perhaps two or three percent versus one percent, but with the numbers of patients we had, that difference was statistically significant.
Track 6
![]() DR LOVE: Would you discuss the AVANT adjuvant study evaluating
FOLFOX and CAPOX with bevacizumab?
DR LOVE: Would you discuss the AVANT adjuvant study evaluating
FOLFOX and CAPOX with bevacizumab?
![]() DR O’CONNELL : AVANT is comparing FOLFOX alone, FOLFOX with
bevacizumab or CAPOX with bevacizumab to learn whether substituting the
oral agent, capecitabine, for the continuous infusion 5-FU/leucovorin will be
as effective (1.3).
DR O’CONNELL : AVANT is comparing FOLFOX alone, FOLFOX with
bevacizumab or CAPOX with bevacizumab to learn whether substituting the
oral agent, capecitabine, for the continuous infusion 5-FU/leucovorin will be
as effective (1.3).
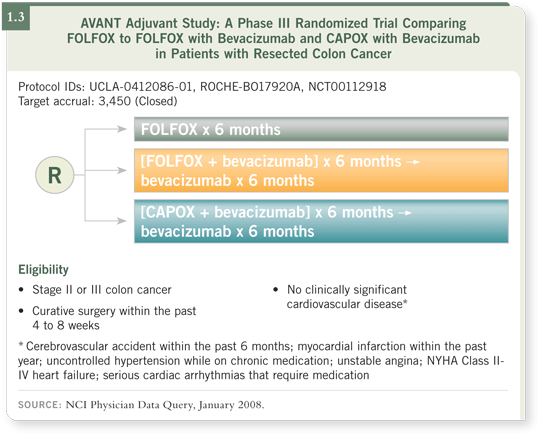
I understand that more than 3,500 patients have entered that trial and that the accrual was recently completed. Like the NSABP-C-08 trial, it’s also using a three-year disease-free survival endpoint, so it will be about three years before we have data from that study.
![]() DR LOVE: Do you believe CAPOX is a reasonable clinical option in the
adjuvant setting right now?
DR LOVE: Do you believe CAPOX is a reasonable clinical option in the
adjuvant setting right now?
![]() DR O’CONNELL : You could make that argument. My impression is that
CAPOX is as effective as FOLFOX in the advanced-disease setting. That was
proved by the NO16966 trial in the metastatic setting that’s been presented on
several occasions (Cassidy 2006). The toxicity profiles are acceptable, and the
efficacy in advanced disease is the same. Yes, I believe this would be a reasonable
option in clinical practice now.
DR O’CONNELL : You could make that argument. My impression is that
CAPOX is as effective as FOLFOX in the advanced-disease setting. That was
proved by the NO16966 trial in the metastatic setting that’s been presented on
several occasions (Cassidy 2006). The toxicity profiles are acceptable, and the
efficacy in advanced disease is the same. Yes, I believe this would be a reasonable
option in clinical practice now.
Track 14
![]() DR LOVE: Can you summarize the ACCENT data you presented at the
2007 ASCO meeting?
DR LOVE: Can you summarize the ACCENT data you presented at the
2007 ASCO meeting?
![]() DR O’CONNELL : The ACCENT database includes clinical data from 18
adjuvant therapy studies. Dan Sergeant, from the Mayo Clinic, is the coordinating
statistician. The goal of the work I presented was to evaluate the
combined data from these studies to determine if the characteristics of the
primary tumor might predict the behavior of the cancer if it recurred months
or years later.
DR O’CONNELL : The ACCENT database includes clinical data from 18
adjuvant therapy studies. Dan Sergeant, from the Mayo Clinic, is the coordinating
statistician. The goal of the work I presented was to evaluate the
combined data from these studies to determine if the characteristics of the
primary tumor might predict the behavior of the cancer if it recurred months
or years later.
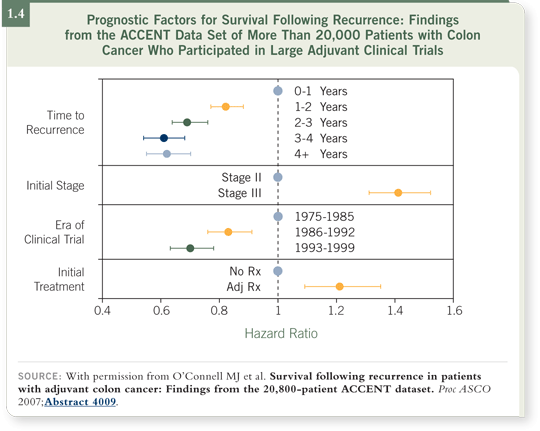
We found that two factors turned out to be strongly prognostic. One of these was stage. Cancer that recurred years after patients with Stage II primary colon cancer had their tumors removed had a much more indolent natural history than the cancer recurring in patients who’d had Stage III cancer. Stage III — node-positive — cancer had a more rapid natural history when it recurred (O’Connell 2007; [1.4]).
What possibilities might explain this? One is that a difference exists in the biology of Stage II versus Stage III disease. If that were the case, you should be able to examine the patients with Stage II and III disease using a molecular analysis and observe different patterns. However, we haven’t observed this type of difference between Stage II and Stage III colon cancer.
![]() DR LOVE: Then how do you explain what’s going on?
DR LOVE: Then how do you explain what’s going on?
![]() DR O’CONNELL : Perhaps patients with Stage III disease are further along in
the natural history of their disease at the time of diagnosis. They may have
more microscopic disease.
DR O’CONNELL : Perhaps patients with Stage III disease are further along in
the natural history of their disease at the time of diagnosis. They may have
more microscopic disease.
The results are profound — in fact, the difference in median survival from the time of diagnosis of metastatic disease, between earlier Stage II and Stage III, was 5.7 months (O’Connell 2007). That’s more than the difference made by any therapeutic regimen in any Phase III trial of chemotherapeutic agents in this disease.
The second major prognostic factor was the time it took for the original cancer to come back. If the tumor recurred quickly — within the first year — the prognosis was poor, suggesting aggressive disease.
However, if the tumor recurred four or five years later, the course of disease tended to be more indolent. We’ve observed this difference in our practices for many years (O’Connell 2007; [1.4]).
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2009 Research To Practice. All Rights Reserved. |