
 |
|||||||

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 6
![]() DR LOVE: What are your thoughts on the six-year follow-up of the
MOSAIC trial presented at the 2007 ASCO meeting, particularly with
regard to stage?
DR LOVE: What are your thoughts on the six-year follow-up of the
MOSAIC trial presented at the 2007 ASCO meeting, particularly with
regard to stage?
![]() DR MARSHALL: We know that a fluoropyrimidine adds a benefit of two to
three percent for the average patient with Stage II disease, but those data from
the MOSAIC trial — which was not designed to evaluate Stage II and Stage
III disease separately — are repeatedly being split and brought back together.
DR MARSHALL: We know that a fluoropyrimidine adds a benefit of two to
three percent for the average patient with Stage II disease, but those data from
the MOSAIC trial — which was not designed to evaluate Stage II and Stage
III disease separately — are repeatedly being split and brought back together.
The message has become confused as to the benefit of adjuvant therapy and the definition of high-risk versus low-risk disease.
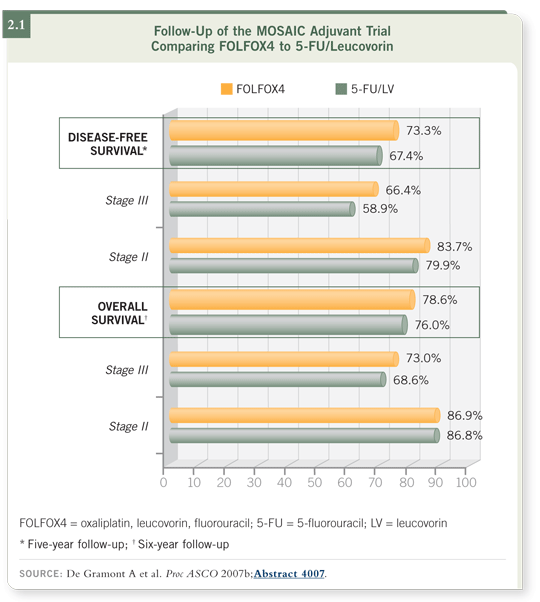
The FDA approved the FOLFOX regimen only for patients with Stage III disease, and we cried foul. Now in the six-year follow-up reported at ASCO, we see these super-split-out data and suddenly we see no disease-free survival benefit with oxaliplatin for patients with low-risk Stage II colon cancer and no overall survival benefit in Stage II disease (de Gramont 2007b; [2.1]).
I applaud NSABP for not revealing the split-out data from NSABP-C-07, the Phase III study of fluorouracil and leucovorin with or without oxaliplatin for patients with Stage II or III colon cancer (Kuebler 2007).
They reported the data in a single set, which was positive for the addition of oxaliplatin. The magnitude of benefit was the same as what was seen in the MOSAIC trial for the overall population.
Track 11
![]() DR LOVE: What are your thoughts on the iBET trial and the question of
whether to continue bevacizumab on disease progression?
DR LOVE: What are your thoughts on the iBET trial and the question of
whether to continue bevacizumab on disease progression?
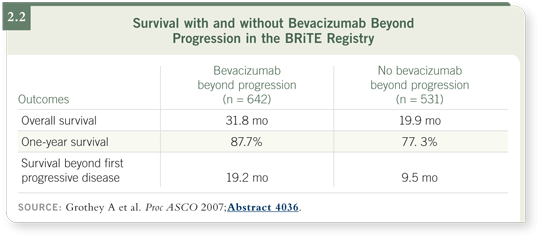
![]() DR MARSHALL: At the 2007 ASCO meeting, Axel Grothey presented data
from the BRiTE registry demonstrating a significant improvement in survival for patients who received bevacizumab beyond progression compared to those
who did not (Grothey 2007; [2.2]), and it’s a strikingly positive finding.
DR MARSHALL: At the 2007 ASCO meeting, Axel Grothey presented data
from the BRiTE registry demonstrating a significant improvement in survival for patients who received bevacizumab beyond progression compared to those
who did not (Grothey 2007; [2.2]), and it’s a strikingly positive finding.
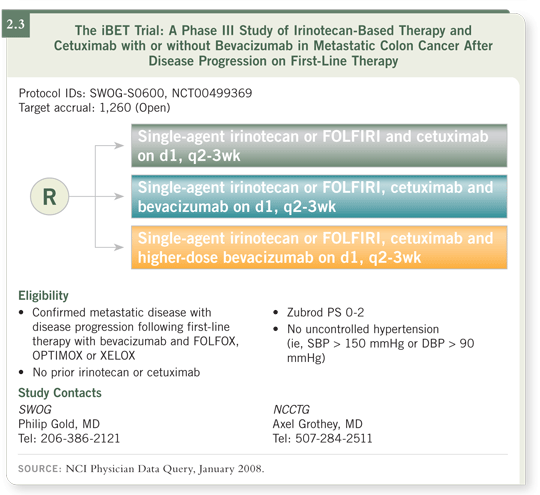
We’re all soft-pedaling the data, using the fact that these are registry data and not from a prospective randomized trial as a rationale for enrolling patients on the iBET trial. Accrual to the trial is slow, and I believe a bias for continuing bevacizumab is already emerging. In addition, two of the three arms in the iBET trial include bevacizumab, and I predict that when the patients in the nonbevacizumab arm develop progression, they’ll receive bevacizumab in the third line, so in the end all the patients will receive bevacizumab beyond progression (2.3).
Track 14
![]() DR LOVE: In our recent Patterns of Care survey, we asked physicians
about how they utilize planned drug “holidays” for patients on FOLFOX
or FOLFIRI with bevacizumab. We found that, whereas 70 percent of the
clinical investigators would continue 5-FU and bevacizumab during the
break, only 29 percent of practicing oncologists do so. They were more
likely to stop all drugs or simply continue with one agent. What do you
think of these findings?
DR LOVE: In our recent Patterns of Care survey, we asked physicians
about how they utilize planned drug “holidays” for patients on FOLFOX
or FOLFIRI with bevacizumab. We found that, whereas 70 percent of the
clinical investigators would continue 5-FU and bevacizumab during the
break, only 29 percent of practicing oncologists do so. They were more
likely to stop all drugs or simply continue with one agent. What do you
think of these findings?
![]() DR MARSHALL: I believe your data reflect the lag involved in getting the
message out. If you had conducted that survey a year earlier, before the
OPTIMOX2 data, I expect the responses would have been more homogeneous,
with everyone feeling comfortable stopping all medications during the holiday.
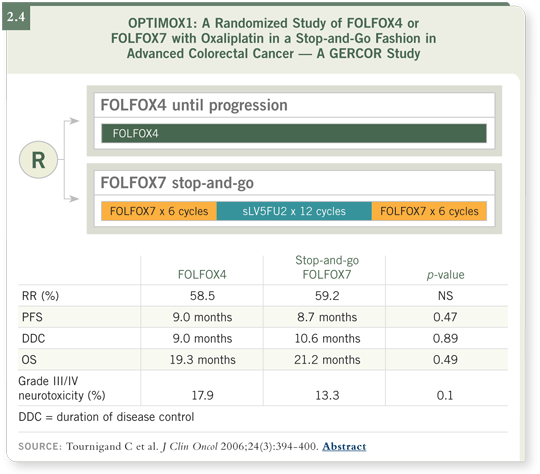
In OPTIMOX1, patients were randomly assigned to continuous FOLFOX or
six cycles of FOLFOX, then 5-FU/leucovorin only, restarting the oxaliplatin
when their disease progressed. The data showed that the latter schedule was as
effective and it was a little less toxic, with less neurotoxicity (2.4).
DR MARSHALL: I believe your data reflect the lag involved in getting the
message out. If you had conducted that survey a year earlier, before the
OPTIMOX2 data, I expect the responses would have been more homogeneous,
with everyone feeling comfortable stopping all medications during the holiday.
In OPTIMOX1, patients were randomly assigned to continuous FOLFOX or
six cycles of FOLFOX, then 5-FU/leucovorin only, restarting the oxaliplatin
when their disease progressed. The data showed that the latter schedule was as
effective and it was a little less toxic, with less neurotoxicity (2.4).
Then the OPTIMOX2 trial, presented at ASCO 2007, compared the winning arm from OPTIMOX1 to a complete holiday — that is, stopping all drugs after six cycles of FOLFOX — and the drugs were not restarted until the disease had regrown to the baseline status. Both a progression-free and an overall survival benefit were seen among the patients who continued to receive 5-FU/leucovorin through the holiday (2.5).
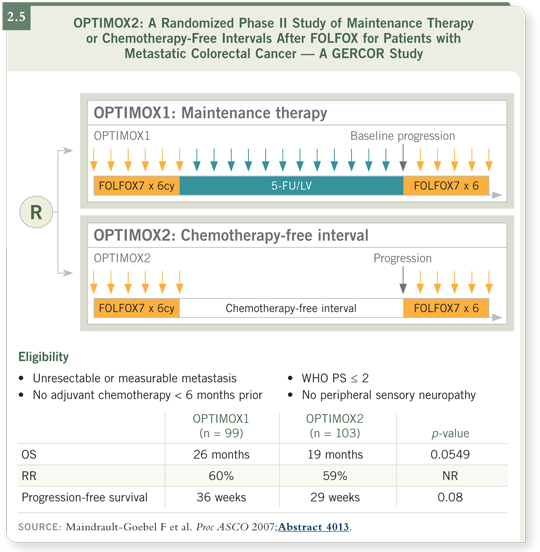
I am still surprised that the OPTIMOX2 data showed that four months or so of 5-FU/leucovorin affected overall survival. However, everyone left ASCO with the message that the patients have to receive something during the holiday. Although the data do indicate that a holiday may be valuable, we need to determine how to optimize that holiday. The data do tell us that waiting until tumors regrow to their baseline size is probably not the right thing to do. Clinically, we restart the chemotherapy at the first sign of progression.
![]() DR LOVE: What is your initial approach in the front-line metastatic setting?
DR LOVE: What is your initial approach in the front-line metastatic setting?
![]() DR MARSHALL: I generally use FOLFOX with bevacizumab, and I reassess
the patients after a few months. When the disease stops responding, I usually
continue the bevacizumab on an every three-week schedule and, for most
patients, I continue 5-FU in the form of capecitabine. My preferred recipe
is continuous, low-dose capecitabine. I also have many patients to whom I
administer every other-week capecitabine right from the beginning.
DR MARSHALL: I generally use FOLFOX with bevacizumab, and I reassess
the patients after a few months. When the disease stops responding, I usually
continue the bevacizumab on an every three-week schedule and, for most
patients, I continue 5-FU in the form of capecitabine. My preferred recipe
is continuous, low-dose capecitabine. I also have many patients to whom I
administer every other-week capecitabine right from the beginning.
| Table of Contents | Top of Page |
| Terms of Use and General Disclaimer | Privacy Policy Copyright © 2009 Research To Practice. All Rights Reserved. |