|
 Home:
Web Guide 2: Richard M Goldberg, MD
Home:
Web Guide 2: Richard M Goldberg, MD
Edited comments by Dr Goldberg
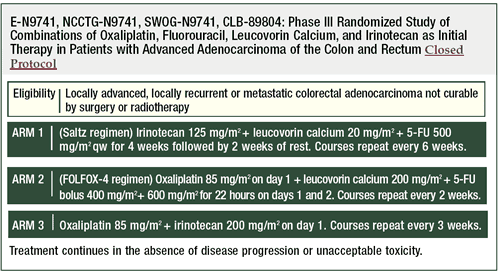
Intergroup trial N9741: Irinotecan/5-FU/leucovorin
vs irinotecan/oxaliplatin vs oxaliplatin/5-FU/leucovorin
This trial originated in 1997. The first version had four arms,
comparing 5-FU/leucovorin (the control arm) to three different ways
of giving 5-FU/leucovorin with irinotecan. By March of 1999, the
NCI became interested in looking at combinations of oxaliplatin
in colon cancer.
It was determined that the most expedient way to test oxaliplatin
in the cooperative group setting was to change N9741 from a four-arm
to a six-arm trial by adding an oxaliplatin plus irinotecan arm
and an oxaliplatin plus 5-FU/leucovorin arm.
The following year several of the arms were closed because of
toxicity. In the arms where bolus 5-FU was given either with irinotecan
or oxaliplatin, there were very high death rates. So, the six-arm
study became a three-arm study. There was a 4.5% 60-day mortality
rate for the patients receiving irinotecan/5-FU/leucovorin (IFL)
and a 1.8% 60-day mortality rate for the patients receiving irinotecan/oxaliplatin
and 5-FU/leucovorin/oxaliplatin (FOLFOX).
The National Cancer Institute convened a group of independent
reviewers to review the charts of the patients who died within 60
days. It was found that about half of the patients died of something
expected, such as dehydration, diarrhea and neutropenia. But the
other half had thrombotic events, such as myocardial infarction,
cerebral vascular accident or mesenteric infarction, which was a
new toxicity syndrome.
Trial results
Roughly 800 patients were enrolled in the trial. They were randomized
to IFL (the new control arm), FOLFOX or irinotecan/oxaliplatin.
The North Central Cancer Treatment Group Data Monitoring Committee
released the results of the trial because the time to progression
and survival statistics surpassed the early stopping rules that
were written into the protocol.
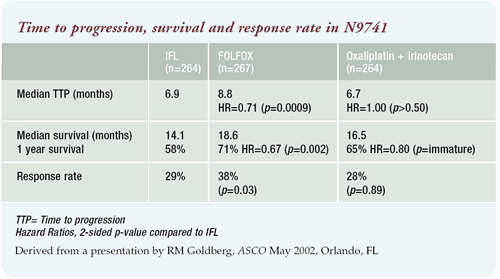
Efficacy
We observed a significant advantage in time to progression (primary
end point), response rate and survival with FOLFOX compared to IFL.
Although a little lower than reported in other trials, a 28% and
29% response rate was observed for irinotecan/oxaliplatin and IFL,
respectively. FOLFOX had a significantly (p = 0.03) higher response
rate of 38%.
The time to progression was 6.9 months with IFL and 6.7 months
with irinotecan/oxaliplatin, compared to 8.8 months with FOLFOX.
Although irinotecan/oxaliplatin and IFL were comparable, there was
a statistically significant (p = 0.0009) difference in time to progression
between FOLFOX and IFL.
These data were consistent with those observed in the Saltz trial
with IFL and the de Gramont trial with FOLFOX. This was the first
randomized comparison of those two regimens.
The survival data was the most interesting; the median survival
was 14.1 months with IFL, 18.6 months with FOLFOX and 16.5 months
with irinotecan/oxaliplatin. The difference between IFL and FOLFOX
was statistically significant (p = 0.002). The number of patients
alive at one year was 58% with IFL, 71% with FOLFOX and 65% with
irinotecan/oxaliplatin. The N9741 trial results confirm the data
from the Saltz trial with IFL and from Aimery de Gramont with FOLFOX-4.
Between 59% and 67% of the patients went on to receive second-line
therapy. Among the patients randomized to IFL, only 17% received
oxaliplatin as second-line therapy. In contrast, 52% of the patients
randomized to FOLFOX went on to be treated with irinotecan, and
45% of the patients randomized to irinotecan/oxaliplatin received
5-FU.
Tolerability of the regimens
More patients receiving FOLFOX withdrew from the trial because
of toxicity compared to those receiving IFL or irinotecan/oxaliplatin.
However, the withdrawals usually occurred late in treatment, after
about eight cycles. Most of the patients on FOLFOX who withdrew
had neurotoxicity while responding to therapy.
In contrast, patients receiving IFL experienced early toxicity
— most often diarrhea, nausea, vomiting and dehydration —
that required either dose reductions or drug discontinuation. Some
patients receiving IFL died early from toxicity. The toxicity associated
with IFL is a little less predictable, occurs earlier and, in my
opinion, is more potentially life-threatening than the toxicity
associated with FOLFOX.
Our trial had a formal quality-of-life analysis, but we have not
been able to analyze it yet. Initially, the toxicity associated
with FOLFOX is less severe than the toxicity with IFL. It is particularly
noticeable with respect to the hair loss, diarrhea and nausea and
vomiting. In my opinion, the statistically significant advantage
seen in toxicity with FOLFOX is also a clinically relevant difference.
Managing patients on irinotecan/5-FU/leucovorin
(IFL)
During the first course of therapy, instead of prescribing four
weekly treatments of IFL and seeing the patients at six weeks, I
now see them weekly. If they have escalating problems with diarrhea,
nausea, vomiting and dehydration, I reduce their dose within the
first cycle of therapy. Reasons to consider either a dose delay
or reduction include incomplete resolution of diarrhea, any signs
of dehydration and borderline or low white blood cell counts.
CASE 1:
36-year-old woman treated with FOLFOX followed by hepatic resection |
History
At her initial diagnosis, this young speech pathologist was
found to have extensive liver metastases, which were not amenable
to resection. She had her primary tumor removed at another
center, and she was given a bleak prognosis. She was asymptomatic
with a performance status of 0.
Follow-up
She enrolled in a clinical trial and was treated with the
FOLFOX regimen.
Case discussion
After about six cycles of FOLFOX, she had a dramatic reduction
in the number and size of her liver lesions. They were surgically
resected, and she is now free of tumor.
She tolerated the therapy very well and was able to continue
working during the treatment. She had minimal neurotoxicity
of the immediate type. Oxaliplatin regimens seldom cause significant
alopecia and have a lower incidence of severe diarrhea, nausea
or vomiting and neutropenia than IFL.
She is just passing her second anniversary since resection,
has no evidence of disease and obviously is very grateful.
She had a six-month-old child at the time of her diagnosis,
and she has been able to watch her toddler grow. |
Neurotoxicity associated with oxaliplatin
There are two kinds of neurotoxicity observed with oxaliplatin.
One occurs within 24 hours of administration and is exacerbated
by cold. For instance, if the patient takes a cold beer out of the
refrigerator the afternoon after their treatment, he/she will feel
like the beer can is electrified. This type of neurotoxicity usually
disappears within a day or two of treatment, but it can occur with
just a few oxaliplatin doses.
The more problematic and long-lasting neurotoxicity usually occurs
after a cumulative dose of about 800 mg/m2 of oxaliplatin. There
is actual damage to the dorsal root ganglion cells. Patients may
have a more prolonged, typical neuropathy, where they have difficulty
buttoning buttons or picking up coins. In contrast to the taxanes
and the vinca alkaloids, oxaliplatin-induced neuropathy improves
over time, but it may not resolve completely.
Aimery de Gramont presented results at ASCO from the MOSAIC trial,
an adjuvant study, which randomizes patients to 5-FU/leucovorin
or 5-FU/leucovorin/oxaliplatin. He found that neuropathy was quite
frequent, particularly in patients receiving their ninth, tenth
and twelfth cycles of oxaliplatin. In most cases the neuropathy
either disappeared or eased dramatically within four months of discontinuing
the treatment.
My clinical observation is that the neuropathy improves over time,
although it remains more problematic than what Dr de Gramont presented.
About 50% of the patients who receive 10 to 12 cycles of oxaliplatin
will have some significant amount of neuropathy, although seldom
severe. Many patients will say, “I want to keep on treatment,
because response is more important to me than this symptom.”
Trials evaluating oxaliplatin in combination
with capecitabine
There are some phase II data that show promising activity for
capecitabine plus oxaliplatin. In the United States, there is some
reluctance to embrace infusional 5-FU regimens, based on the need
for an indwelling catheter and the potential for catheter-related
complications. It would be ideal if we had randomized trial data
demonstrating that an oral drug could replace infusional 5-FU.
Sequence of therapy for advanced colorectal cancer
An interesting presentation at ASCO by Axel Grothey compared the
Mayo Clinic regimen of 5-FU/leucovorin to FOLFOX. He demonstrated
an advantage in survival, response rate and time to progression
for FOLFOX compared to 5-FU/leucovorin.
He also looked at the six most recent randomized trials presented
at ASCO in advanced colon cancer and demonstrated that survival
increases when patients have access to oxaliplatin, irinotecan and
5-FU. In the Saltz trial, only about five percent of the patients
received oxaliplatin as second-line therapy, and the median survival
was just over 14 months. In the European trials where oxaliplatin
and irinotecan were available as second-line therapy, the median
survival ranged between 19 and 21 months.
The optimal therapy for patients with advanced colon cancer includes
all three drugs (5-FU, irinotecan and oxaliplatin). How they should
be sequenced is not yet known, but sequencing is less important
than the availability of all three drugs.
Selected references
|

