|
 Home:
Web Guide 2: Edward Lin, MD
Home:
Web Guide 2: Edward Lin, MD
Edited comments by Dr Lin
Role of COX-2 in colorectal cancer
Cyclooxygenase-2 (COX-2), a regulator of inflammation, is also
known as the central “playmaker” in tumor angiogenesis.
COX-2 is expressed in colorectal cancers, and its expression may
be a negative prognostic factor. Furthermore, COX-2 is a very potent
angiogenic factor, and it is upstream of VEGF (vascular endothelial
growth factor) and PDGF (platelet-derived endothelial growth factor).
The exact role of COX-2 in colorectal cancer progression is still
being evaluated. However, it is an important target for colorectal
cancer treatment.
Phase II trial of chemotherapy in combination
with celecoxib for metastatic colorectal cancer
Dr Charles Blanke conducted a phase II trial with triple therapy
plus a COX-2 inhibitor (irinotecan/5-FU/leucovorin plus celecoxib)
in patients with previously untreated, unresectable or metastatic
colorectal cancer. His data suggests a potential improvement in
irinotecan-related diarrhea and an increase in vascular syndromes.
My interpretation is that the COX-2 inhibitors potentially work
through the antiangiogenic pathway. This may be the reason the patients
had increased vascular syndromes, such as myocardial infarctions
or strokes.
Capecitabine in combination with celecoxib
Capecitabine is activated through thymidine phosphorylase (TP),
also known as a platelet-derived endothelial growth factor, which
is a very potent angiogenic enzyme. Tumor cells upregulate and turn
on the angiogenic phenotype. Potentially, capecitabine delivers
more cytotoxic drug to the tumor cells than normal cells.
Although the combination of capecitabine/celecoxib must be evaluated
in prospective clinical trials, the idea is to give two drugs that
potentially antagonize their side effects, but also enhance their
antitumor activity and improve the delivery of the drug to the tumor.
Etiology of hand-foot syndrome
Hand-foot syndrome only occurs rarely with bolus 5-FU. Other drugs
also cause hand-foot syndrome, such as liposomal doxorubicin and
docetaxel. Hand-foot syndrome is drug-related — if you do
not give the drug, you do not get hand-foot syndrome.
Because of the way capecitabine is metabolized and where it accumulates,
I believe hand-foot syndrome is probably due to its metabolites.
In fact, in our prospective trial, we will look at COX-2 levels
as well as try to determine which metabolites accumulate in the
hands and feet. I think the metabolites are probably prostaglandin-like.
The feet seem to have delayed circulation, so there might be a
difference in how the metabolites are cleared. Additionally, enzyme
expression may vary in different tissues. The hands and feet, like
tumor cells, may potentially accumulate more 5-FU. I am not aware
of any data demonstrating that patients who develop hand-foot syndrome
have a better tumor response. I think hand-foot syndrome is one
of the toxicities highly specific to patients who are receiving
continuous-infusion 5-FU, capecitabine and some other drugs.
Celecoxib attenuates capecitabine-induced hand-foot
syndrome
We thought the hand-foot syndrome might be driven by COX-2, which
has been “proven” to be the central regulatory mediator
of inflammation. We had also observed that patients taking celecoxib
together with capecitabine did not experience any hand-foot syndrome.
Retrospective analysis of patients receiving
capecitabine/celecoxib
In a case-controlled retrospective analysis, capecitabine-treated
patients with tumor-related pain were given celecoxib (200 mg twice
a day) and observed for hand-foot syndrome. Then they were compared
to a historical-control group of patients primarily treated with
capecitabine alone and not given any other NSAID. Both groups of
patients were identified by the pharmacy database and matched according
to the capecitabine dose. Most of the patients received 1,000 mg/m2
of capecitabine twice a day. The median ages for the capecitabine/celecoxib
and control groups were 65 and 55, respectively. Therefore, the
control group was a bit younger.
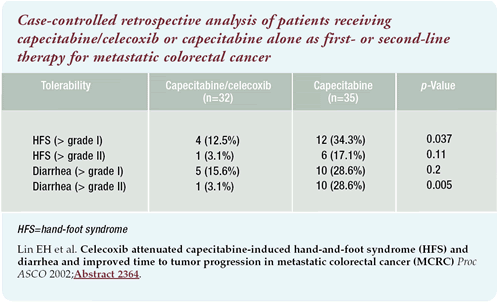
Tolerability
Six months ago when the data was gathered, the capecitabine/celecoxib
cohort consisted of about 32 patients. In those patients receiving
capecitabine/ celecoxib, grade III hand-foot syndrome was almost
nonexistent. Only one patient developed grade III hand-foot syndrome
while taking celecoxib.
That patient had bilateral DVTs and congestive heart failure with
grade III edema at baseline. He actually developed more of a foot
syndrome, but no hand syndrome. Other than that patient, we saw
very mild hand-foot syndrome — minor erythema and minor pain
that did not interfere with the patients’ daily work. In the
capecitabine-only, dose-matched control group, 34% and 17% experienced
greater than grade I and grade III hand-foot syndrome, respectively.
Another interesting observation was related to diarrhea. Approximately
28% of the patients receiving capecitabine alone required hospitalization
for hydration. We rarely encountered the need for hydration in the
patients taking capecitabine/celecoxib. There were, however, a couple
of patients who developed dehydration, but they required only outpatient
hydration.
Additionally, the patients receiving celecoxib seemed to have
much better pain control. About 30% of the patients had a performance
status of 2, but they all universally improved when given celecoxib
along with capecitabine.
Efficacy
Although quite a few patients had failed prior chemotherapy (i.e.,
irinotecan, oxaliplatin and prior 5-FU regimens), capecitabine produced
a decrease in CEA and disease stabilization. In the patients who
had received prior irinotecan and were treated with capecitabine/celecoxib,
there were two partial responses. No partial responses were seen
in any irinotecan-experienced patients who were treated with capecitabine
alone. However, three chemotherapy-naïve patients had a partial
response to capecitabine alone, which lasted about nine months.
In contrast, seven patients who received capecitabine/celecoxib
had responses that lasted for about 12 months. One patient has been
on therapy for approximately 18 months.

Phase II capecitabine/celecoxib trial
The retrospective data are hypothesis-generating and the basis
for our large prospective phase II trial that will evaluate full-dose
capecitabine with a higher dose of celecoxib. The primary objectives
of that trial will be to achieve better tumor response and provide
a cohort for comparison to a historical cohort with respect to the
dose intensity of capecitabine, quality of life and the incidence
of the hand-foot syndrome. The first phase of clinical testing will
evaluate the combination for feasibility, toxicity, tolerance, quality
of life and clinical benefit.
Capecitabine/celecoxib has the advantage of being a totally oral
regimen. Many patients treated with both drugs have maintained a
very good quality of life. Patients with a good response have been
able to go back to their daily routine activities. To prove the
initial observation and hypothesis, our phase II trial will focus
on the hand-foot syndrome and tumor efficacy. Then we may consider
adding an EGFR (epidermal growth factor receptor) antagonist and
“pilot” a targeted-therapy combination.
Capecitabine/celecoxib in clinical practice
Our results were very consistent, and we have treated five or
six patients who developed capecitabine-induced hand-foot syndrome
with celecoxib (200 mg twice a day). Universally, their hand-foot
syndrome has improved.
Recently, we had a patient on a capecitabine/irinotecan trial
who developed grade III hand-foot syndrome. Despite reducing the
dose of capecitabine from 1,000 mg/m2 to 750 mg/m2 twice a day,
she still had hand-foot syndrome. At 500 mg/m2 twice day she did
not have hand-foot syndrome, but her tumor progressed.
An option for her was to increase the dose of capecitabine to
900 mg/m2 twice a day and initiate celecoxib. She actually did well
with that option, and the hand-foot syndrome did not return. Of
the patients treated with celecoxib for the hand-foot syndrome,
four or five have not experienced a recurrence while being able
to maintain the capecitabine dose intensity.
Selected references
|

