You are here: Home: CCU 3 | 2005: Paulo M Hoff, MD
| Paulo M Hoff, MD |
EDITED COMMENTS |
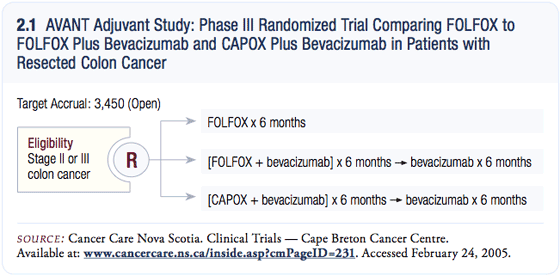
 AVANT adjuvant trial: FOLFOX versus FOLFOX plus bevacizumab versus CAPOX plus bevacizumab AVANT adjuvant trial: FOLFOX versus FOLFOX plus bevacizumab versus CAPOX plus bevacizumab
This very large multinational trial will attempt to accrue 3,450 patients (2.1). The study utilizes two dosing levels for bevacizumab. Since the CAPOX (capecitabine and oxaliplatin) regimen uses an every three-week infusion of oxaliplatin, those patients will receive 7.5 mg/kg of bevacizumab every three weeks for six months.
The patients treated with FOLFOX will receive five mg/kg of bevacizumab every two weeks for six months. Hence, the dose intensity is the same. Once the patients finish their chemotherapy, they will receive 7.5 mg/kg of bevacizumab every three weeks.
A lot went into the discussions for this trial. One issue was the choice of a control arm. While some discussion remains, we ultimately opted for FOLFOX. The next discussion was about patient selection. We decided to conduct the trial in patients with Stage III and high-risk Stage II disease, although the patients with high-risk Stage II disease will be part of an exploratory analysis.
The final and perhaps greatest discussion we had was about the length of treatment. Right now, the majority of adjuvant trials incorporate six months of chemotherapy and one year of molecularly targeted agents. We followed the same lead.
The rationale for using bevacizumab alone for six additional months after chemotherapy comes from the thought that bevacizumab has enough activity by itself to suppress the formation of new blood vessels and inhibit tumor growth.
Of course, it is possible that most of the benefit from bevacizumab comes from its association with chemotherapy. ECOG-E3200 demonstrated that bevacizumab alone was inferior to FOLFOX with or without bevacizumab (Giantonio 2005). Since this is a question without a good answer, the feeling was that it was justified to use the molecularly targeted agents longer than the chemotherapy. I see this as a proof-of-concept trial, and the length of treatment will have to be further refined in future trials.
Role of adjuvant CAPOX in the nonprotocol setting
There is great interest, especially in the community, in having an oral chemotherapy-based regimen, and the CAPOX regimen is very attractive in that regard. Given the opportunity, patients will tend to choose oral agents. We have the X-ACT adjuvant study showing that capecitabine was equivalent and had a hint of being better than bolus 5-FU/leucovorin (Cassidy 2004). I think the data from the Phase II CAPOX trials in the advanced setting are intriguing enough to say that it’s at least equivalent to FOLFOX.
I wouldn’t recommend CAPOX as my first option in the adjuvant setting, because obviously we prefer to use evidence-based medicine. However, I would not necessarily find it incorrect to use CAPOX in the adjuvant setting. Scientifically, it makes sense. Depending on individual patient issues (eg, dealing with access lines, etcetera), it might make sense.
Nonprotocol adjuvant therapy for patients with colon cancer
If a patient has Stage III colon cancer, I tend to offer adjuvant FOLFOX first, unless they have a contraindication such as pre-existing peripheral neuropathy from other causes or a dependence on fine motor skills to earn a living. For example, I had a patient who worked with small watches who opted to receive capecitabine alone. I still tend to discuss adjuvant FOLFOX with patients who have contraindications, and if they want it, I will still use it.
Once I explain all the options, even some patients in whom I would prefer to use FOLFOX surprisingly ask to receive capecitabine. They feel attracted to the oral agent. Also, in patients with severe comorbid conditions or the very frail elderly patients, I tend to use adjuvant capecitabine instead of FOLFOX.
For those patients who present with Stage II disease, the decision about the use of adjuvant chemotherapy is much more complicated. Obviously, we have to discuss the potential benefits and toxicities of chemotherapy. I tend to offer adjuvant chemotherapy more strongly if their disease has a high-risk feature (eg, obstruction, perforation or lymphovascular invasion).
Adjuvant therapy for elderly patients with colon cancer
I don’t consider age, per se, a contraindication to chemotherapy. I think that’s a problem these days; patients are sometimes denied chemotherapy when it could be beneficial. We have to remember that if a patient is an 82-year-old now and in good shape, they still have a life expectancy of several years.
I think adjuvant chemotherapy will be beneficial for those patients, and I don’t necessarily approach them differently than a younger patient. However, I tend to be more conservative with the choice of chemotherapy. I tend to discuss the options, and I’m more inclined to use a fluoropyrimidine alone in the adjuvant setting.
Quite frankly, even before the X-ACT trial results, I was already inclined to use adjuvant capecitabine on occasion in elderly patients. That was not necessarily evidence-based medicine, but we had data from the metastatic setting showing it was equivalent to 5-FU. Capecitabine makes a huge difference for the patients’ convenience and ability to receive the treatment at home.
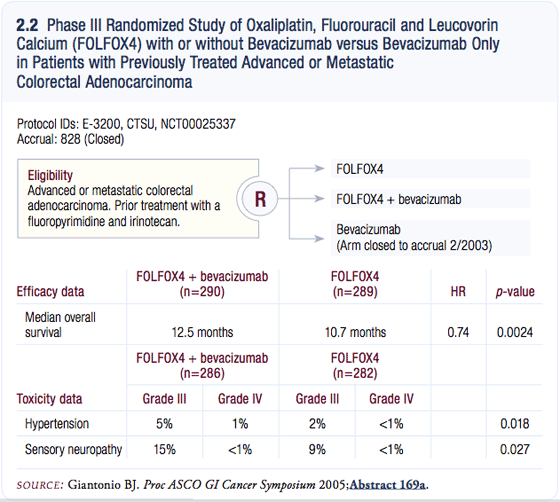
E3200: FOLFOX with or without bevacizumab
E3200 randomly assigned patients who had failed front-line treatment with IFL (irinotecan/bolus 5-FU/leucovorin) to one of three treatment arms: FOLFOX, FOLFOX plus bevacizumab or bevacizumab alone.
In the first interim analysis, bevacizumab alone was inferior to the other two treatments, and accrual to that arm was suspended. As you would imagine, the results have been eagerly awaited, because this is the first trial that we investigated the combination of bevacizumab and FOLFOX — a main first-line regimen used in the United States.
When the initial toxicity results were reported, we were impressed that patients receiving FOLFOX plus bevacizumab had a higher incidence of Grade III peripheral neuropathy (Mitchell 2005). There were two possibilities: (1) bevacizumab when added to FOLFOX caused peripheral neuropathy or (2) patients were staying on treatment longer.
Of course, we all wanted the second possibility to be the true reason. That was confirmed at the end of 2004 when an NCI news release reported approximately two months of benefit in the overall survival for those patients who had received bevacizumab plus FOLFOX. Median overall survival was about 10.5 months with FOLFOX alone, and around 12.7 months with FOLFOX plus bevacizumab (Mitchell 2005; [2.2]).
Continuation of bevacizumab as part of second-line therapy
One big question arising from E3200 to which we do not have an answer is: If you treat a patient with an irinotecan-based regimen and bevacizumab as frontline therapy and the patient responds, but eventually progresses, do you treat with FOLFOX alone or FOLFOX plus bevacizumab as second-line therapy? Does it help to maintain the patient on bevacizumab? We do not know, and I would be sad if we did not conduct a trial to find out. I think its time to back off and perform a trial evaluating the continuation of bevacizumab.
If you assume that the main mechanism of bevacizumab is a decrease in intratumoral pressure and that when the tumor progresses it is because of resistance to chemotherapy and not to bevacizumab, then bevacizumab should help again if combined with effective second-line therapy. If that is incorrect and bevacizumab has direct activity and the tumors are resistant to that direct activity, then the trial would be negative, but I believe the trial will most likely be positive.
Select publications
 |
| Dr Hoff is an Associate Professor of Medicine at The University of Texas MD Anderson Cancer Center in Houston, Texas. |
|

