You
are here: Home: CCU 1 | 2003:
Niall Tebbutt, BM, Bch, PhD, MRCP, FRCP
 |
|
 |
| Niall
Tebbutt, BM, Bch,
PhD, MRCP, FRCP |
 |
|
 |
Consultant
Medical Oncologist,
Austin-Repatriation Medical
Centre,
Melbourne, Australia |
|
|
|
 |
|
|
 |
Edited comments by Dr Tebbutt
Phase III study comparing capecitabine
with fluorouracil and oxaliplatin with cisplatin in patients
with advanced esophagogastric cancer
Trial background, rationale
and design
This study builds upon the epirubicin/cisplatin/5-FU (ECF)
regimen devised by David Cunningham's group in the GI units
of the Royal Marsden Hospital. Importantly, this regimen uses
a protracted venous infusion (PVI) schedule of 5-FU, which
is not as myelosuppressive as bolus schedules of 5-FU.
The rationale for the ECF regimen was that, while these
three agents don’t have single-agent activity in the
adjuvant setting for esophagogastric cancer, by putting them
together you’ve got three agents with independent activity
and nonoverlapping toxicity profiles. This regimen went through
initial Phase II trials, showing significant activity. There
have now been two large randomized studies establishing ECF
as a highly active regimen, which is a standard of care in
the United Kingdom, many parts of Europe and Australia.
This study examines the role of capecitabine and oxaliplatin
in bringing the ECF regimen forward. Capecitabine is an oral
treatment, without the hassles and complications of lines
and pumps. The other potential advantage of capecitabine is
the fact that it is activated via thymidine phosphorylase,
which itself is upregulated by other agents. So, there is
potential for synergy by combining capecitabine with other
agents, which activate thymidine phosphorylase.
Oxaliplatin has activity in a variety of cisplatin-resistant
tumors, and generally has a more favorable toxicity profile
than cisplatin. There is less renal toxicity, and patients
do not need the same prehydration with oxaliplatin that they
need with cisplatin. There is also less auditory toxicity
and neurotoxicity.
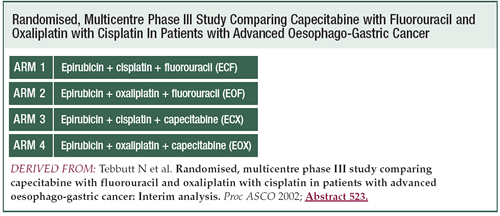
The study was designed to establish the role of those two
agents — capecitabine and oxaliplatin — building
from the ECF regimen. It's a 2-by-2 factorial design, with
essentially two randomizations: cisplatin or oxaliplatin,
and PVI 5-FU or capecitabine.
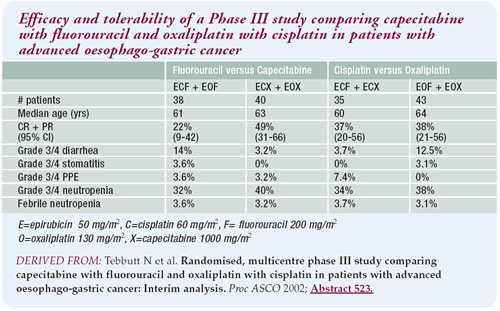
Interim study results
Currently accrual stands at about 200 patients, with a targeted
accrual of 600. I presented a planned interim analysis after
80 patients, but it’s difficult to make significant
interpretations. All the regimens, and importantly the two
experimental regimens, look active. The study is designed
to compare the 5-FU treatment arms with the capecitabine treatment
arms separately. Response rates and toxicity results in the
two experimental arms are very promising.
The toxicity results were no worse for the capecitabine
treatment arms, and they certainly looked better than the
toxicity encountered with PVI 5-FU. But, this is a randomized
Phase II trial, and we can’t interpret too much. When
the study is completed, we will be able to very confidently
define the role of capecitabine and oxaliplatin in upper GI
cancer.
Select publications of clinical
trial results in advanced esophagogastric cancer
|

